Antibody-Drug Conjugates (ADCs) are one of the fastest-developing treatments in the field of oncology, with 15 ADCs already approved and over 210 currently undergoing clinical trials. In recent years, the development of ADC drugs has entered a flourishing phase, especially demonstrating significant efficacy and good safety profiles against targets such as HER2, EGFR, Trop2, CLDN18.2, and Nectin-4, with remarkable results from combination therapies and bispecific ADCs, providing patients with more treatment options. With continuous technological advancements and innovations, the application prospects of ADC therapies in cancer treatment are very promising.
ADC Drugs: The “Magic Bullets” in Cancer Treatment
Antibody-drug conjugates (ADCs) are referred to as the “magic bullets” of cancer treatment. They are a new type of biotherapeutic drug that links monoclonal antibodies to cytotoxic drugs via chemical linkers. The core concept lies in combining the specific targeting ability of antibodies with the powerful killing action of cytotoxic drugs. Once in the bloodstream, the antibody portion of the ADC can specifically recognize and bind to the target antigens on the surface of tumor cells, forming an ADC-target antigen complex, which is then internalized into the cell via endocytosis. Inside the cell, the linker undergoes cleavage under specific intracellular conditions, releasing the cytotoxic drug, thereby killing the tumor cells. This design aims to achieve precise targeting of tumor cells, minimize damage to normal cells, and enhance the efficacy and safety of cancer treatment.
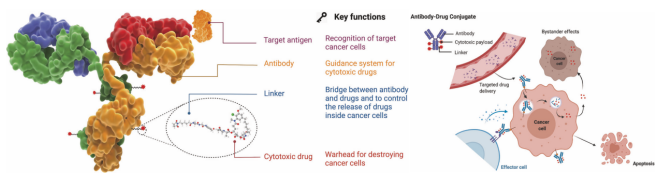
Figure 1. Structural Characteristics and Mechanism of Action of ADC Drugs
Developing ADC drugs requires comprehensive consideration of multiple key factors. The first is target selection, which should focus on high specificity expression in tumor cells and relevance to tumor biological processes; in terms of antibody design, it is necessary to ensure low immunogenicity, strong specificity, high internalization efficiency, and long half-life to achieve effective delivery of ADCs in the body; linker design should balance stability and cleavability, as well as chemical compatibility, while avoiding ADC aggregation to prevent premature release of the active payload in the bloodstream and ensure precise release of the active drug at the target site; cytotoxic drugs must demonstrate stability under physiological conditions and have functional groups that can bind to antibodies; aside from selecting antibodies, linkers, and cytotoxic drugs, the method of attaching small molecule components to antibodies is a key element for successfully constructing ADCs, and choosing the appropriate conjugation method significantly impacts the stability and efficacy of ADCs.
Review of the Development History of ADC Drugs: A Legend of Anticancer Drug Development Amid Challenges
The concept of ADCs was first proposed by German physician and Nobel laureate Paul Ehrlich in the early 20th century. However, it was not until the 1970s, with the advent of monoclonal antibodies and the maturation of recombinant protein engineering technology, that ADC research gradually became a hot area in the development of antibody drugs. In 2000, Mylotarg, developed by Wyeth Pharmaceuticals, received accelerated approval from the FDA for the treatment of CD33-positive acute myeloid leukemia (AML) patients, becoming the world’s first commercial ADC drug. However, due to fatal toxicities observed in clinical studies, this drug was withdrawn from the market in 2010, but was re-approved for sale in 2017 after specification adjustments and additional clinical evidence were provided. Since then, the ADC field has experienced a long period of stagnation.
During these eight years, only four new products, Adcetris, Kadcyla, Besponsa, and Lumoxiti, were approved, and the entire ADC market grew slowly. 2019 marked a significant turning point in the history of ADC development. In that year alone, three ADCs, Polivy, Padcev, and Enhertu, were approved. Among them, Enhertu, developed in collaboration between Daiichi Sankyo and AstraZeneca, is seen as the “biggest catalyst for the dramatic recovery of the ADC field.” Enhertu achieved groundbreaking success in the Destiny-Breast series of studies, significantly improving survival and remission rates for breast cancer patients. Additionally, due to Enhertu’s significant activity against both HER2-positive and low-expressing tumors, the industry has paid more attention to the “bystander effect” of ADCs on malignant cells outside the target. After 2019, the ADC pipeline welcomed a surge of approvals for products such as Trodelvy, Blenrep, and Zynlonta, with the market size also experiencing exponential growth.
However, the ADC drug market also faces some challenges, such as the termination of collaborations between companies and potential drug resistance issues. Nevertheless, overall, the growth trend is evident, and the market potential is enormous, making the development in cancer treatment and related fields worth looking forward to.
Intense Competition for ADC Drug Targets
Who Will Stand Out?
Since the approval of the first HER2 monoclonal antibody, the HER2 target has received significant attention in the field of tumor treatment, undergoing over twenty years of development. Currently, the approved HER2 ADC drugs include trastuzumab emtansine, trastuzumab deruxtecan, and vedotin, with numerous other HER2 ADC drugs in clinical research stages, such as SHR-A1811 from Hengrui Medicine and DP303c from CSPC Pharmaceutical Group. Among these drugs, trastuzumab deruxtecan (DS-8201, Enhertu) has become a significant breakthrough in the HER2 ADC field due to its unique structure and high anti-tumor activity (Figure 3).
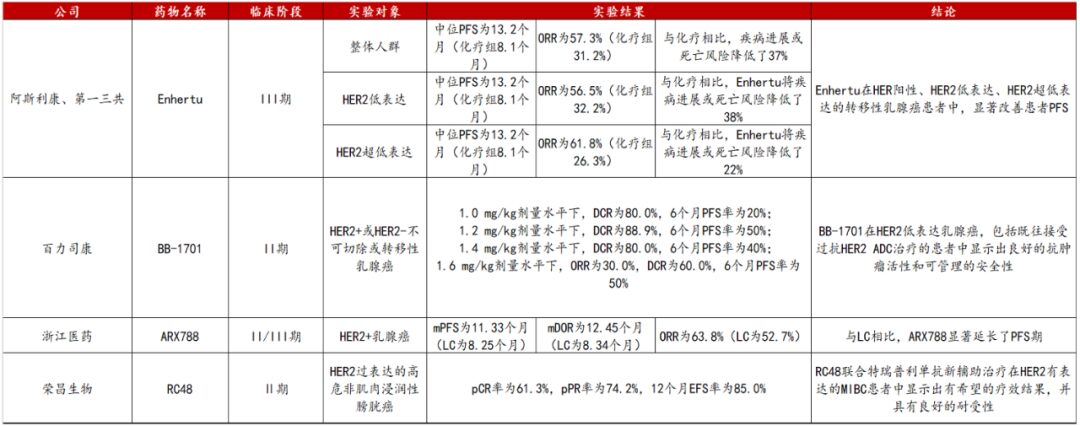
Figure 3. Latest HER2 Target ADC Drug Data from ASCO 2024
DS-8201 is a humanized HER2-targeted ADC drug jointly developed by Daiichi Sankyo and AstraZeneca. Since its first approval in 2019, this drug has been approved for marketing in multiple countries and regions, including the United States, Japan, the European Union, and China, with indications covering HER2 positive breast cancer, HER2 positive gastric cancer, HER2 low-expressing breast cancer, HER2 mutation non-small cell lung cancer, and HER2 positive solid tumors. As the approved indications and regions continue to expand, the sales of trastuzumab deruxtecan have also been increasing year by year. In 2023, the global sales of trastuzumab deruxtecan reached approximately 395.9 billion yen (about 30 billion USD), a year-on-year increase of about 78% (Figure 4).
Figure 4. Clinical Trial Layout and Global Sales Revenue of DS-8201
Trop2 ADC has a multifaceted development status in the field of lung cancer. In terms of efficacy, products from different companies show varying efficacy in different subpopulations. The products from Daiichi Sankyo and Kelun-Biotech perform well in EGFR positive and non-squamous carcinoma populations but are limited in effectiveness for EGFR wild-type or squamous carcinoma patients; Gilead’s product shows good efficacy in populations with driving gene mutations, with comparable effectiveness in both non-squamous and squamous carcinomas. Gozatuzumab, SKB264, and Dato-DXd lead in global development progress. There are significant differences in clinical layouts among companies. Kelun-Biotech/Merck has many clinical trials based on EGFR mutations, PD-L1 expression levels, and standard clinical therapies for lung cancer populations. Daiichi Sankyo/AstraZeneca also has a considerable number of trials, with the first phase three clinical trial not distinguishing between gene and protein markers, and a broader layout for combination therapies, while Gilead has fewer trials (Figure 5).
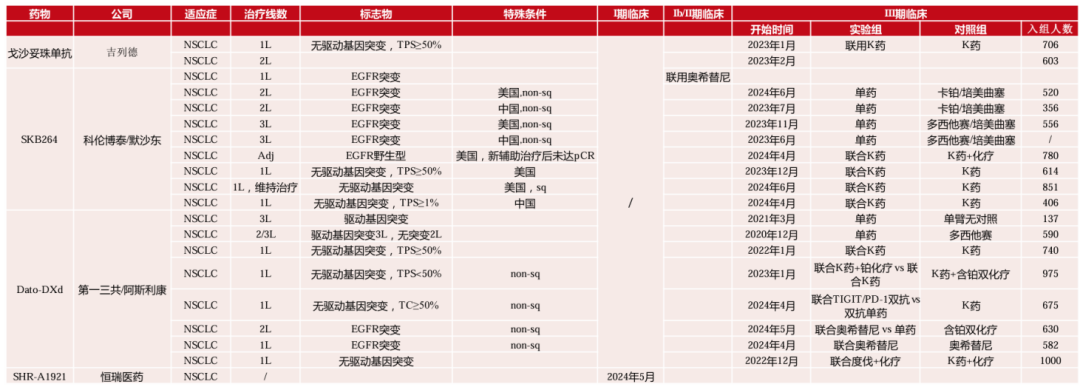 Figure 5. Development Progress of Trop2 ADC Drugs
Figure 5. Development Progress of Trop2 ADC Drugs
At the 2024 ASCO conference, preliminary data from Kelun-Biotech’s phase II clinical study OptiTROP-Lung01 showed promising results, with SKB264 combined with PD-L1 monoclonal antibody demonstrating excellent performance in first-line treatment of driving gene-negative NSCLC, achieving a median PFS of 15 months in the low-dose group, which is more advantageous compared to other drugs, highlighting the great potential of SKB264 in combination with tumor immunotherapy drugs in the NSCLC field.
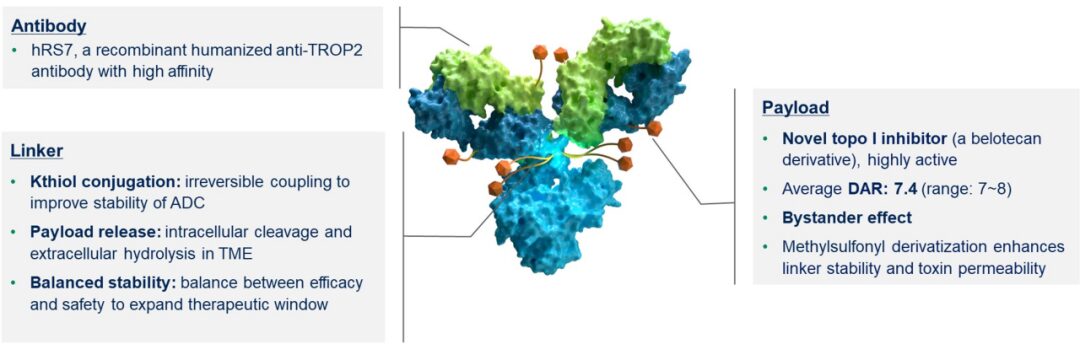
Figure 6. Structure of SKB264
Nectin-4 ADC has made significant progress in development as a highly promising tumor treatment drug (Figure 7). Internationally, Seagen’s Padcev is an early Nectin-4 ADC drug that has made breakthroughs in the treatment of urothelial carcinoma. However, Padcev has issues such as skin toxicity, which presents improvement directions for subsequent development. Domestic companies have demonstrated strong innovative capabilities in the development of Nectin-4 ADCs. Mawei Biotech and CSPC Pharmaceutical Group have adopted advanced third-generation ADC technologies. This technology significantly enhances the uniformity of the drug and reduces side effects through optimization of the drug conjugation process.
Represented by Mawei Biotech’s 9MW2821 and CSPC’s SYS6002, they have achieved better efficacy and safety using unique technologies. The IDDC technology used in 9MW2821 and the transaminase technology employed in SYS6002 have contributed to the enhancement of drug performance. These technologies allow the drugs to target tumor cells more precisely, enhancing their tumor-killing ability while minimizing damage to surrounding normal cells, further improving treatment outcomes and patient tolerance. Overall, the development trend of domestic Nectin-4 ADCs is very promising. With ongoing research and development, they are expected to demonstrate their therapeutic value in more indications. This will not only provide new treatment options for more cancer patients but also further enhance the status of domestic drugs in the international cancer treatment field and promote the improvement of global cancer treatment standards.
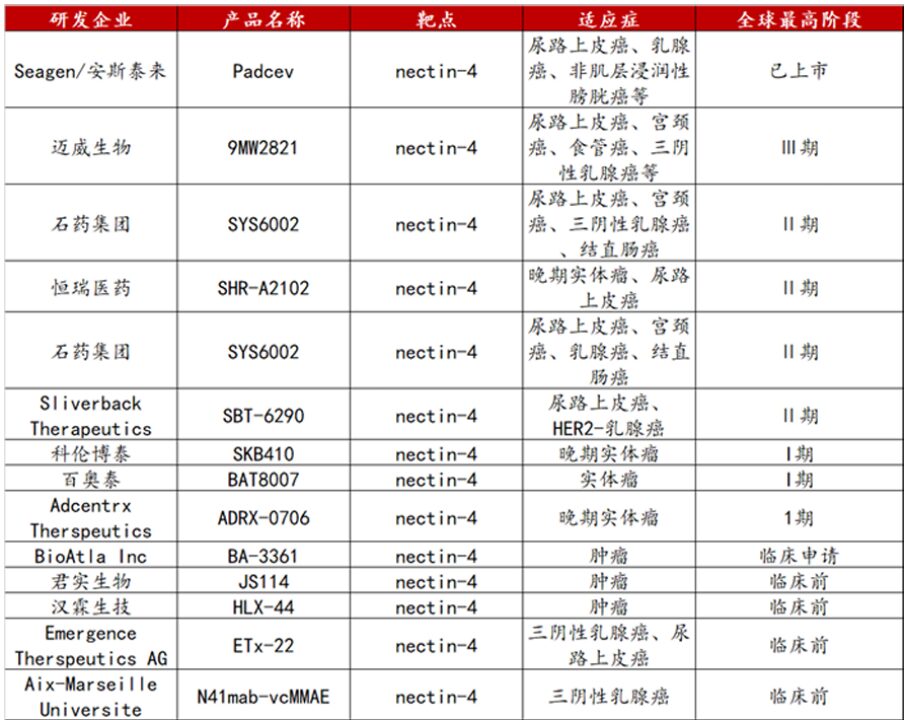
Figure 7. Development Progress of Nectin-4 Target ADC Drugs
Rapid Development Progress of CLDN18.2 ADC (Figure 8). Many domestic pharmaceutical companies are actively participating, including IBI343 from Innovent Biologics, CMG901 developed in collaboration between CanSino and Lepu Biopharma, and LM-302 from Lixian Pharmaceuticals, which are all at relatively late clinical research stages. For instance, IBI343 has been included in the breakthrough therapy designation list, CMG901 has received multiple international recognitions and clinical data showing considerable objective response rates and disease control rates, and LM-302 has also shown good results in studies across different cancer indications. Additionally, relevant products from companies such as Hengrui, CSPC, DQ, and Jianxin are also actively conducting clinical research, with a wealth of clinical data continually emerging to provide a basis for subsequent research and drug optimization, promising new hope for cancer treatment.
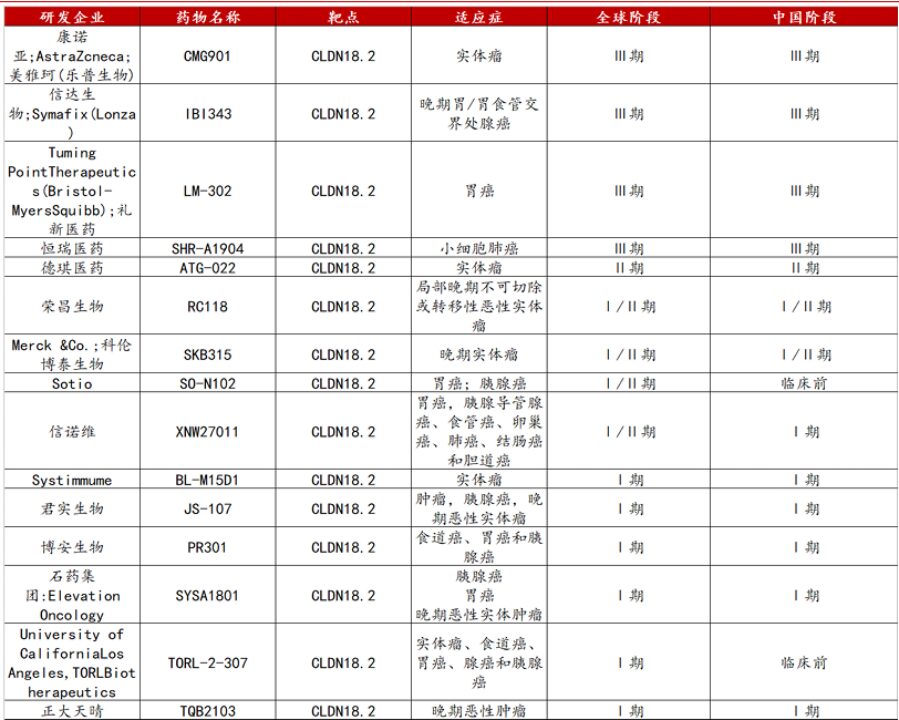
Figure 8. Development Progress of CLDN18.2 Target ADC Drugs
Integration of Multiple Technologies to Reshape the Landscape of Cancer Treatment
With the rapid approval of oncology-directed ADC drugs, future developments may continue along existing paths, including the research and development of more ADC drugs carrying TOPO1 inhibitors, microtubule inhibitors, and DNA damaging agents, testing already approved ADC drugs in unexplored indications, and evaluating combinations of ADCs with immune checkpoint inhibitors and other anticancer agents. At the same time, to realize the dream of the “magic bullet,” further innovative designs of ADC drugs are needed. This includes fine-tuning the molecular characteristics of ADCs, such as selecting cytotoxic molecules with different potencies and mechanisms of action, adjusting the drug-to-antibody ratio, and optimizing linking technologies to enhance drug activity and reduce toxicity.
Additionally, ADC drug performance can also be optimized through the combined use of other drugs or new delivery strategies. More radical innovative ideas include replacing one or more components of ADCs, such as developing bispecific or multispecific ADCs, introducing peptide masking, modifying antibody binding characteristics, and using smaller molecules to replace antibodies. Despite the significant progress ADC drugs have made, our understanding of their mechanisms of action remains limited. Future research needs to delve deeper into the processes of ADC action in vivo, including drug uptake, distribution, metabolism, and excretion, as well as interactions between drugs and targets and other biomolecules. This will help us better design and optimize ADC drugs, improving their therapeutic effects and safety.
References
[1] Fu, Zhiwen et al. “Antibody drug conjugate: the “biological missile” for targeted cancer therapy.” Signal transduction and targeted therapy vol. 7,1 93. 22 Mar. 2022, doi:10.1038/s41392-022-00947-7
[2] Colombo, Raffaele et al. “The Journey of Antibody-Drug Conjugates: Lessons Learned from 40 Years of Development.” Cancer discovery, OF1-OF20. 23 Oct. 2024, doi:10.1158/2159-8290.CD-24-0708
[3] Wenfeng Fang, et al. 2024 ASCO Oral 8502.
[4] China Postal Securities, Southwest Securities, Zhihuiya Research Report
Scan the QR code to add the editor of the Biopharmaceutical Circle, and eligible individuals can join
Biopharmaceutical WeChat Group!
Please specify: Name + Research Direction!
All articles reprinted by this public account are for the purpose of conveying more information and are clearly marked with sources and authors. Media or individuals who do not wish to be reprinted can contact us ([email protected]), and we will immediately process the deletion. All articles only represent the author’s views and do not represent the position of this site.




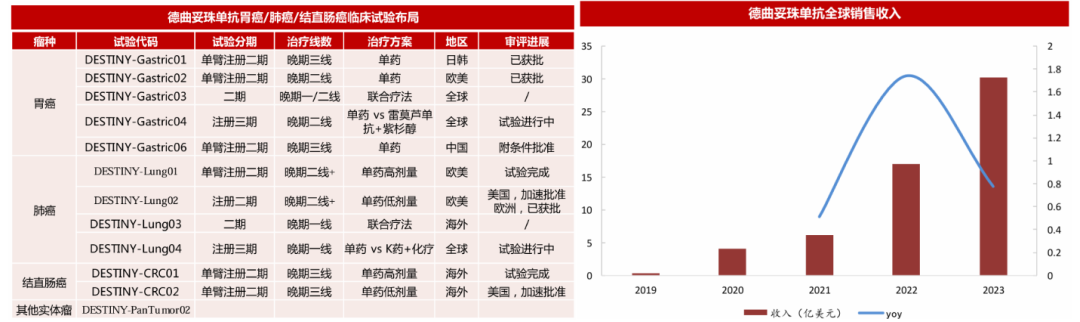
 Figure 5. Development Progress of Trop2 ADC Drugs
Figure 5. Development Progress of Trop2 ADC Drugs




