
 Click the blue text to follow us Literature Review
Click the blue text to follow us Literature Review

This week’s shared literature is a study published on January 8, 2025, by Zhikai Mai from the Foshan Maternal and Child Health Hospital in the journal Molecular Cancer (IF: 27.7; Q1 in Zone 1), titled “Intra-tumoral sphingobacterium multivorum promotes triple-negative breast cancer progression by suppressing tumor immunosurveillance”.
 Background
Background
Breast cancer is the most common cancer among women worldwide, with an increasing incidence, especially among younger women. The tumor microbiome is considered an important regulatory factor in the tumor microenvironment (TME). Dysbiosis (i.e., imbalance in bacterial composition and metabolic activity) plays a significant role in the development and progression of breast cancer. However, the effects of specific intra-tumoral bacteria on tumor progression and their mechanisms remain unclear.
The article analyzed cancerous and adjacent tissues from breast cancer patients through 16S rDNA gene sequencing, identifying a bacterium with high abundance in the tumor tissues of breast cancer patients—Sphingobacterium multivorum (S. multivorum). This bacterium is a Gram-negative aerobic organism and an opportunistic human pathogen associated with various severe infections. The study aims to explore the impact of S. multivorum on breast cancer progression and its potential mechanisms, hoping to provide new targets and strategies for breast cancer treatment.
 Results
Results
1.16S rDNA gene sequencing analysis of cancerous and adjacent tissues in breast cancer patients
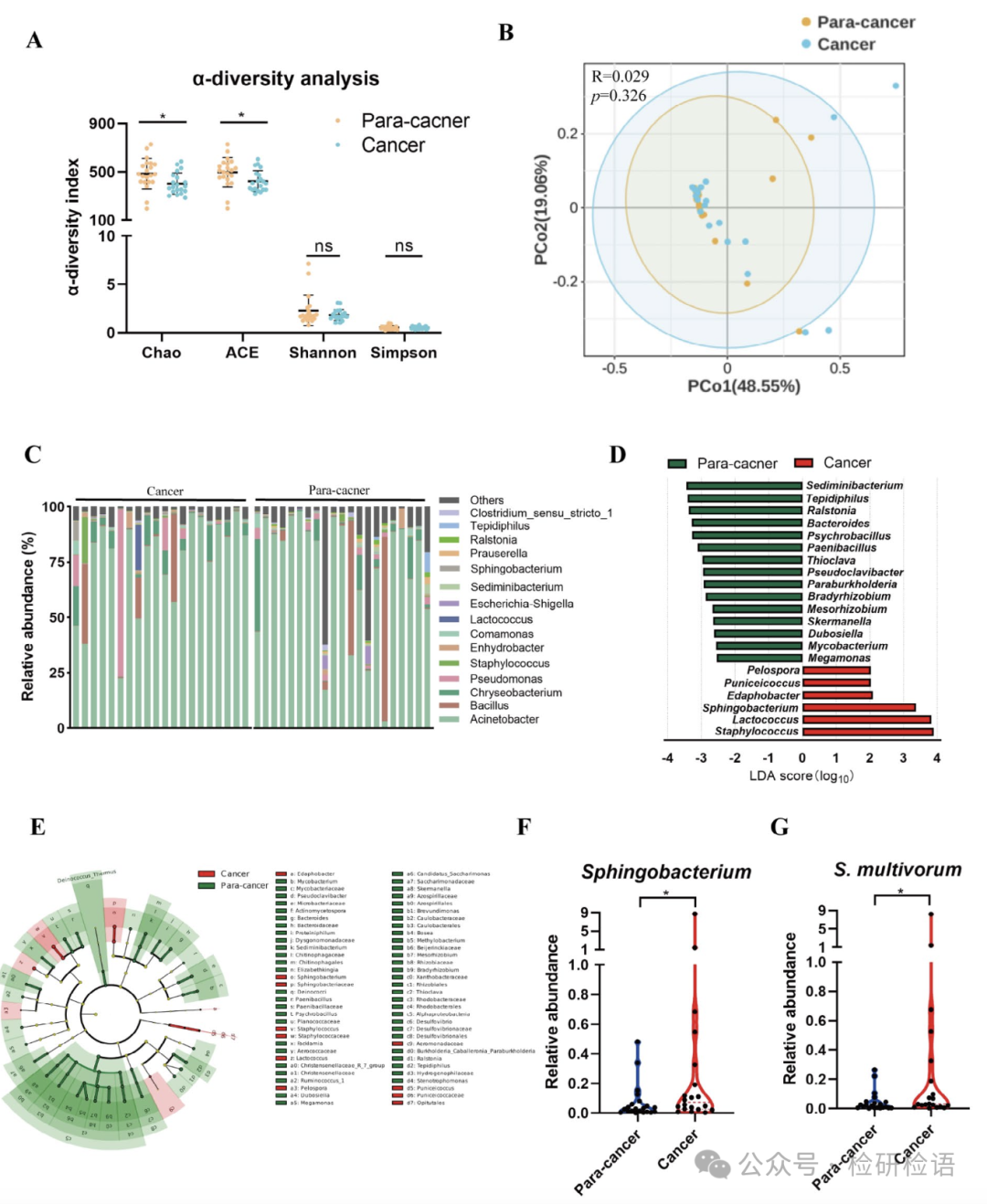
To assess the correlation between different tumor-resident bacteria and breast cancer development, Figure 1 presents the 16S rDNA gene sequencing analysis of cancerous and adjacent tissues from breast cancer patients. The relative abundance and homogeneity of microbial communities in cancerous and adjacent tissues were evaluated using Chao1, ACE, Shannon, and Simpson indices (Figure 1A). Chao1 and ACE values reflect the species richness of bacterial communities, while Shannon and Simpson values reflect species diversity. The results showed significant differences in Chao1 and ACE indices between cancerous and adjacent tissues, while no significant differences were observed in Simpson and Shannon indices. Principal coordinate analysis (PCoA) comparing the microbial community structures of cancerous and adjacent tissues revealed significant enrichment of Sphingobacterium in cancerous tissues (Figure 1B). The bar chart shows that the relative abundance of Sphingobacterium ranked 11th among 170 bacterial genera (Figure 1C). Linear discriminant analysis effect size (LEfSe) analysis indicated that Staphylococcus, Lactococcus, Sphingobacterium, and Edaphobacter were more abundant in tumor tissues, while Sediminibacterium, Tepidiphilus, Ralstonia, and Bacteroides were more abundant in adjacent tissues (Figure 1D-E). Wilcoxon non-parametric test analysis revealed significant enrichment of Sphingobacterium (especially S. multivorum) in breast cancer tissues (Figure 1F-G).
2. Intra-tumoral S. multivorum accelerates tumor growth
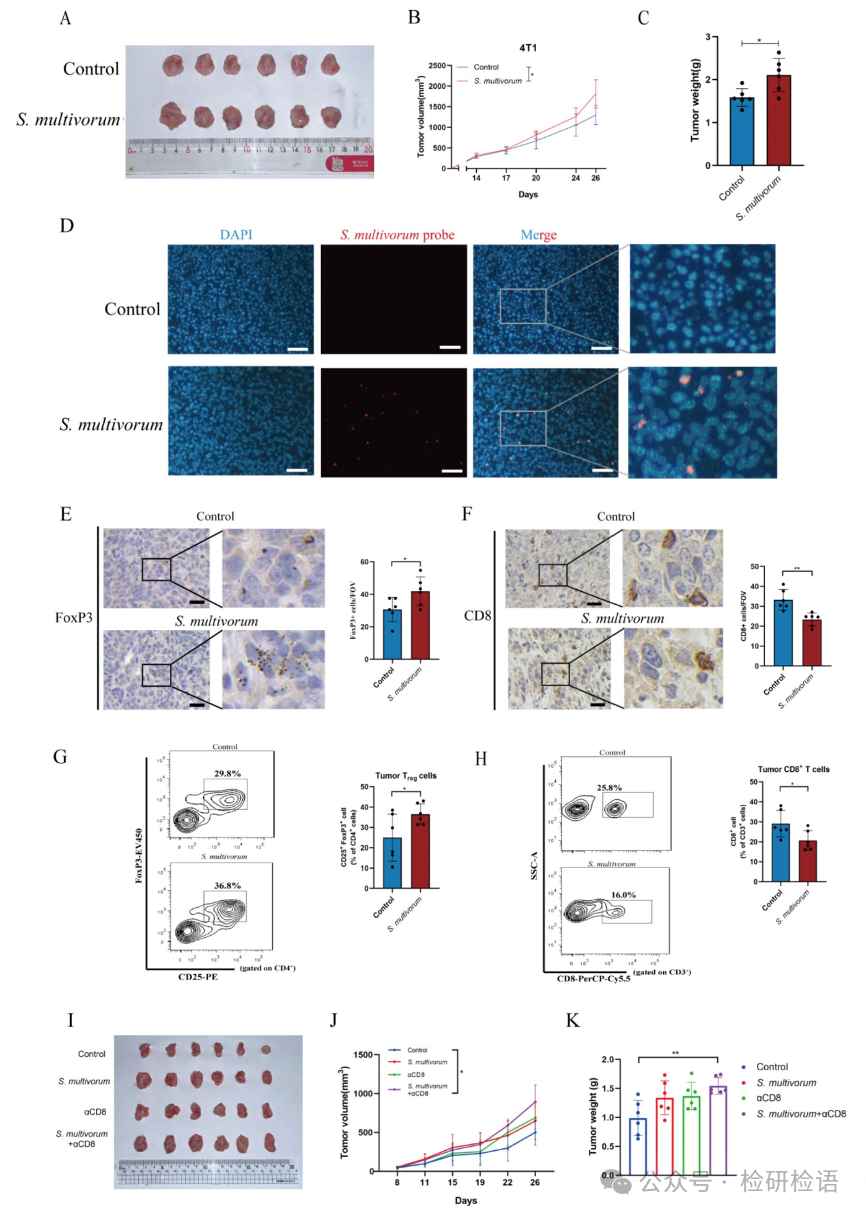
Given that S. multivorum is one of the most abundant bacteria in the tumor tissues of breast cancer patients, the article hypothesized that S. multivorum may promote breast cancer progression. The effect of S. multivorum on tumor growth was evaluated using a 4T1 tumor mouse model (Figure 2A). Compared to the control group, mice injected with S. multivorum showed significant increases in tumor volume (Figure 2B) and weight (Figure 2C). Fluorescence in situ hybridization (FISH) detected S. multivorum in the tumor tissues of mice, with positive signals observed only in the tumor tissues of mice injected with S. multivorum, confirming the colonization of S. multivorum in the tumor (Figure 2D). Immunohistochemical analysis showed increased infiltration of FoxP3+ Treg cells in the S. multivorum group (Figure 2E), while CD8+ T cell infiltration decreased (Figure 2F). Flow cytometry further validated the increase of Treg cells (Figure 2G) and the decrease of CD8+ T cells (Figure 2H). CD8+ T cell depletion experiments indicated that the absence of CD8+ T cells enhanced the tumor-promoting effect of S. multivorum (Figure 2I-K).
3.S. multivorum inhibits the anti-tumor effect of PD-1 antibodies
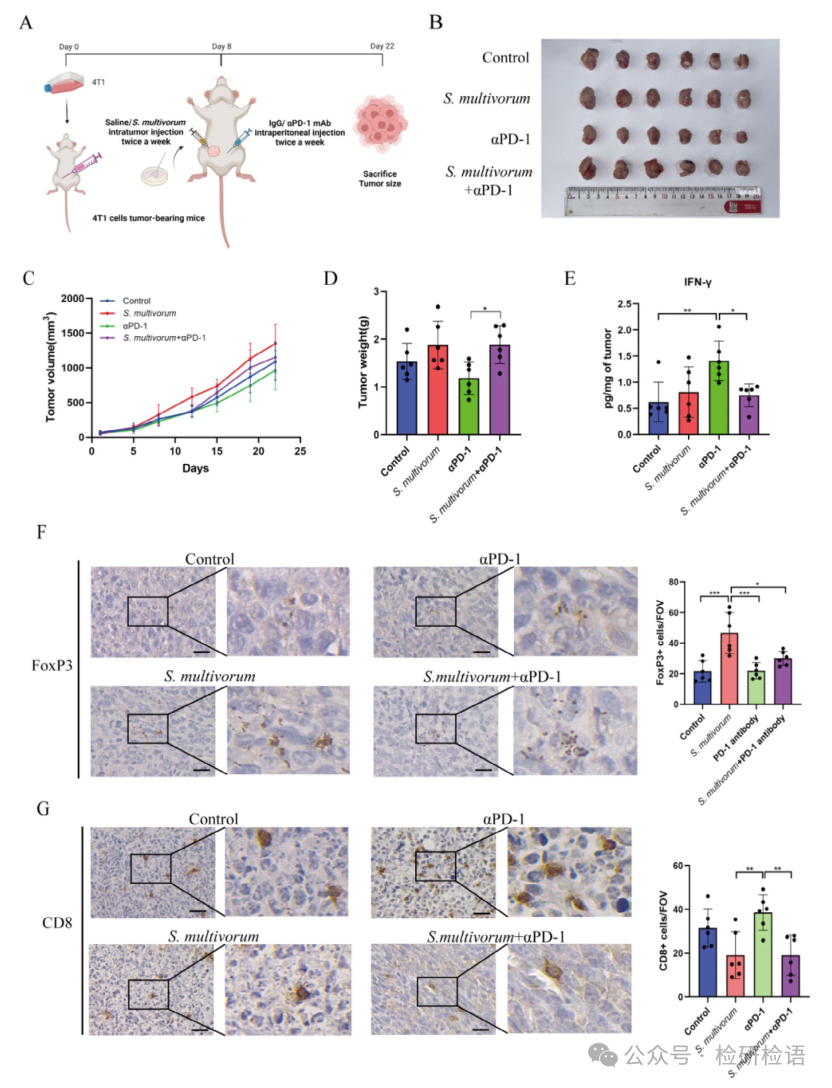
In mice treated with αPD-1 mAb, the tumor-promoting effect of S. multivorum was further confirmed. S. multivorum or saline was injected into the tumors of 4T1 tumor mice, combined with intraperitoneal injection of IgG or αPD-1 mAb (Figure 3A). The results showed that S. multivorum significantly weakened the therapeutic effect of PD-1 antibodies, as evidenced by increased tumor volume (Figure 3B) and weight (Figure 3C-D). Immunohistochemical analysis revealed upregulation of FoxP3 expression in tumors of mice injected with S. multivorum (Figure 3F). Compared to mice receiving only αPD-1 mAb treatment, those receiving combined treatment with S. multivorum and αPD-1 mAb showed reduced levels of IFN-γ in tumor tissues (Figure 3E) and decreased infiltration of CD8+ T cells (Figure 3G). This indicates that the intra-tumoral colonization of S. multivorum not only accelerated breast cancer growth but also weakened the therapeutic response to αPD-1 mAb.
4.S. multivorum reduces propionylcarnitine to inhibit tumor growth
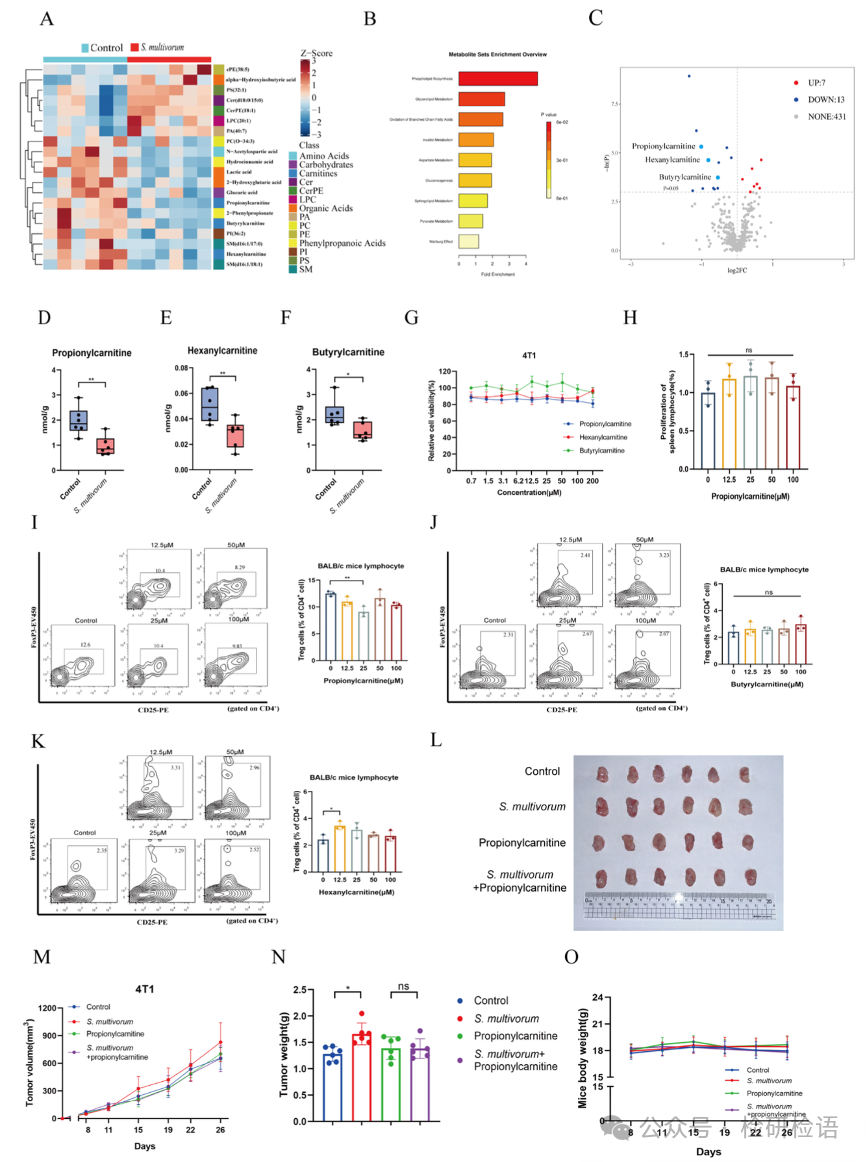
In the tumor tissues of mice injected with S. multivorum, targeted metabolomics analysis identified changes in metabolites, with a total of 451 different metabolites identified, of which 20 showed significant differences (Figure 4A). Among these, 7 metabolites increased in abundance, while 13 decreased (Figure 4B-C). Three classes of carnitine metabolites (propionylcarnitine, hexanoylcarnitine, and butyrylcholine) were significantly reduced in the S. multivorum group (Figure 4D-F). In vitro experiments indicated that propionylcarnitine, hexanoylcarnitine, and butyrylcholine did not directly affect tumor cell proliferation (Figure 4G), but propionylcarnitine could reduce the proportion of Treg cells in splenic lymphocytes (Figure 4H-I), while hexanoylcarnitine and butyrylcholine did not have this effect (Figure 4J-K). In vivo experiments confirmed that injection of S. multivorum alone could promote tumor growth, while the group injected with both propionylcarnitine and S. multivorum did not show significant increases in tumor growth, indicating that propionylcarnitine may inhibit tumor growth by regulating the proportion of Treg cells (Figure 4L-O).
5.S. multivorum induces immune suppression by enhancing CCL20 and CXCL8 expression
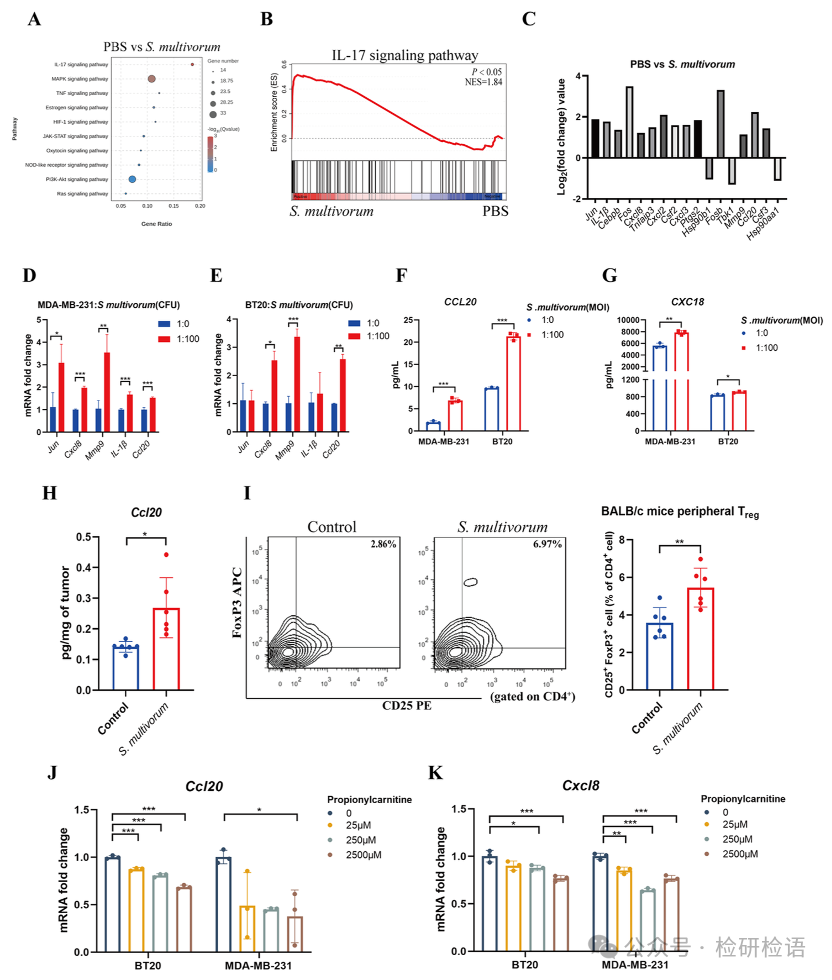
To investigate the molecular mechanisms by which S. multivorum promotes breast cancer growth, RNA sequencing analysis was performed on MDA-MB-231 cells stimulated by S. multivorum. The results showed that S. multivorum activated the IL-17 signaling pathway (Figure 5A-B). Differential gene analysis revealed significant upregulation of genes such as Jun, Fos, IL-1β, CXCL8, MMP9, and CCL20 (Figure 5C), which was validated by qPCR (Figure 5D-E). ELISA tests confirmed that S. multivorum significantly increased the levels of CCL20 and CXCL8 secreted by MDA-MB-231 and BT20 cells (Figure 5F-G). In the 4T1 tumor mouse model, CCL20 levels (Figure 5H) and the proportion of Treg cells in peripheral blood (Figure 5I) were significantly increased in tumor tissues of mice injected with S. multivorum. Furthermore, propionylcarnitine could inhibit the expression of CCL20 and CXCL8 (Figure 5J-K).
6.Potential mechanisms by which S. multivorum promotes breast cancer growth
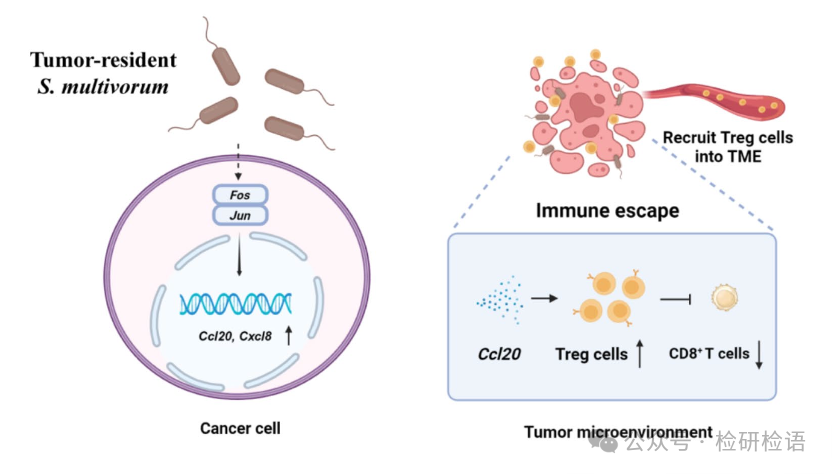
A schematic diagram summarizes the mechanism of action of S. multivorum (Figure 6): After colonizing the tumor, S. multivorum promotes tumor cells to secrete CCL20 and CXCL8. CCL20 recruits Treg cells and suppresses CD8+ T cell infiltration, creating an immunosuppressive microenvironment that promotes tumor growth and immune evasion. Meanwhile, S. multivorum reduces propionylcarnitine levels, further weakening the anti-tumor immune response.
 Conclusion
Conclusion
The article reveals the mechanism by which the endogenous bacterium S. multivorum promotes breast cancer progression by suppressing tumor immuno-surveillance. S. multivorum promotes the secretion of CCL20 by tumor cells, recruits Treg cells, and reduces CD8+ T cell infiltration, thereby forming an immunosuppressive tumor microenvironment. Additionally, S. multivorum lowers the levels of propionylcarnitine, further facilitating tumor immune evasion.
The article not only reveals the important role of S. multivorum in breast cancer progression but also proposes propionylcarnitine as a potential therapeutic target. Future research could further explore how to enhance the efficacy of immunotherapy by regulating the intra-tumoral microbiome and metabolites, providing more effective treatment strategies for breast cancer patients.
Finally, here is the link to the full text:https://pmc.ncbi.nlm.nih.gov/articles/PMC11708301/END
This concludes the content of this literature sharing.In the upcoming posts, our team will continue to focus on sharing the latest literature. We look forward to your attention~