Nitrogen dioxide (NO₂) is a major nitrogen oxide (NOx) pollutant produced by the combustion of fossil fuels, posing significant threats to the ecosystem and human health, necessitating effective detection methods to monitor NO₂. Current NO₂ detection technologies have limitations in stability and selectivity.
According to reports from Maims Consulting, a research team led by Professor Yang Minghui at Dalian University of Technology has developed a transition metal nitride sensor that exhibits excellent selectivity for NO₂, with a sensitivity 30 times greater than the strongest interfering gas, NO. The sensor maintains stability for over six months without the use of platinum (Pt) or other precious metals. This outstanding performance is achieved by preparing highly active titanium nitride (TiNx) nanoparticles with an ultra-large surface area and a high concentration of nitrogen vacancies. In contrast, commercial TiN samples show no gas sensing activity. The sensor has potential scalability for everyday NO₂ detection and demonstrates that robust, high-performance gas sensors based on inexpensive metal nitrides (without precious metals) are promising candidates for environmental monitoring technologies. Relevant research findings were published in Nature Communications under the title “Titanium nitride sensor for selective NO₂ detection.”
In this work, the researchers’ strategy was to synthesize TiNx through high-temperature ammonia decomposition of the metal-organic framework (MOF) precursor MIL-125(Ti). The resulting TiNx exhibited the highest surface area and high levels of nitrogen vacancies (up to 20%) reported among transition metal nitrides, thus achieving excellent sensor performance without using Pt. The MOF-derived TiN-600 (synthesized at a temperature of 600℃) nanoparticles exhibited a high specific surface area of 221.9 m²/g, which is 90 times that of the commercially available TiN-CM sample. Furthermore, experiments confirmed the substantial formation of nitrogen vacancy centers in the TiN-600 material, which is closely related to its gas-sensitive response.
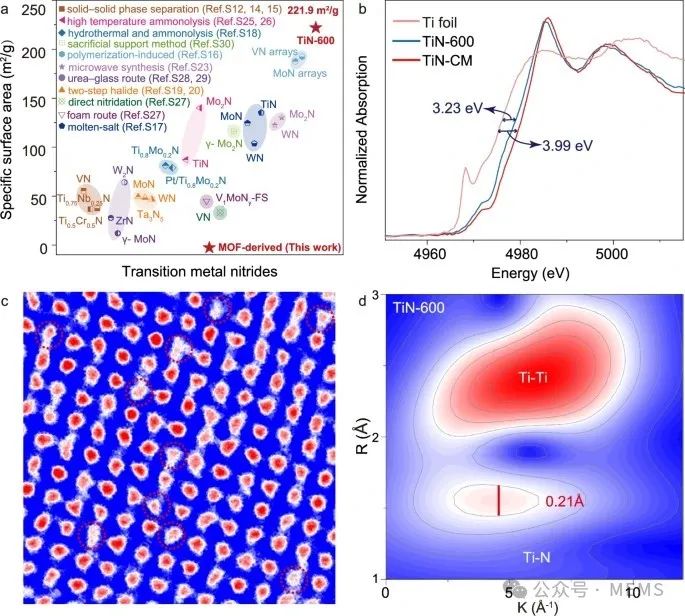 MOF-derived TiNx with high specific surface area and rich nitrogen vacancies
MOF-derived TiNx with high specific surface area and rich nitrogen vacancies
TiN-600 shows the highest response to NO₂, with response and recovery times of 7.4 seconds and 5.2 seconds, respectively. The TiN-600 sensor exhibits a positive linear response to NO₂ in the range of 50 ppb to 50 ppm, with a sensitivity of 0.12 μA/ppm and a detection limit (LoD) as low as 50 ppb, capable of detecting the minimum concentration set by the U.S. Environmental Protection Agency (EPA) (53 ppb).
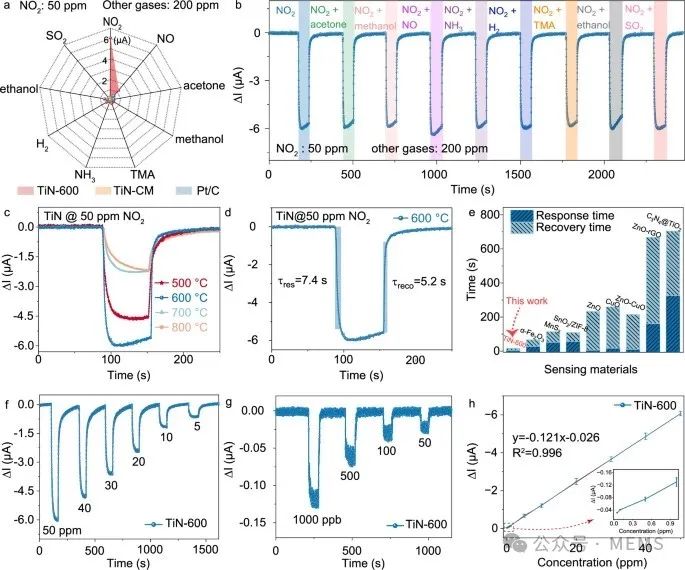 Selectivity, response-recovery characteristics, and response-concentration correlation of TiNx sensor
Selectivity, response-recovery characteristics, and response-concentration correlation of TiNx sensor
Considering the practical application of the TiN-600 gas sensor, the researchers conducted a six-month dynamic sensing response monitoring. The results showed that the response decreased by only 4.2% compared to the first day, indicating that the TiN-600 sensor exhibits excellent long-term stability.
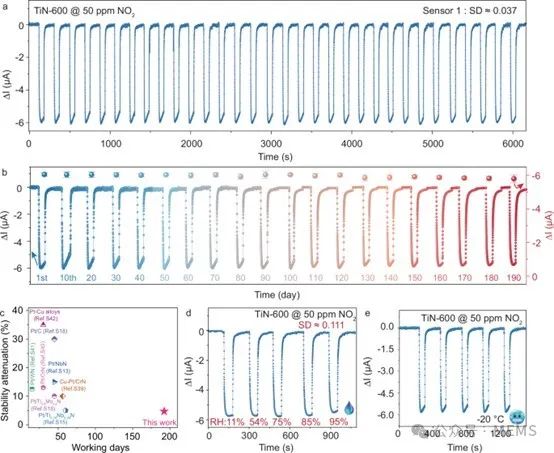 Stability and environmental tolerance of TiNx sensor
Stability and environmental tolerance of TiNx sensor
The in situ monitoring of nitrogen vacancies in TiN-600 is crucial for understanding the adsorption mechanism of the material. The researchers utilized in situ Fourier-transform infrared spectroscopy (FTIR) and electron paramagnetic resonance (EPR) methods to demonstrate that the NO₂ sensing mechanism occurs through the reduction sequence of NO₂→NO₂⁻→NO.
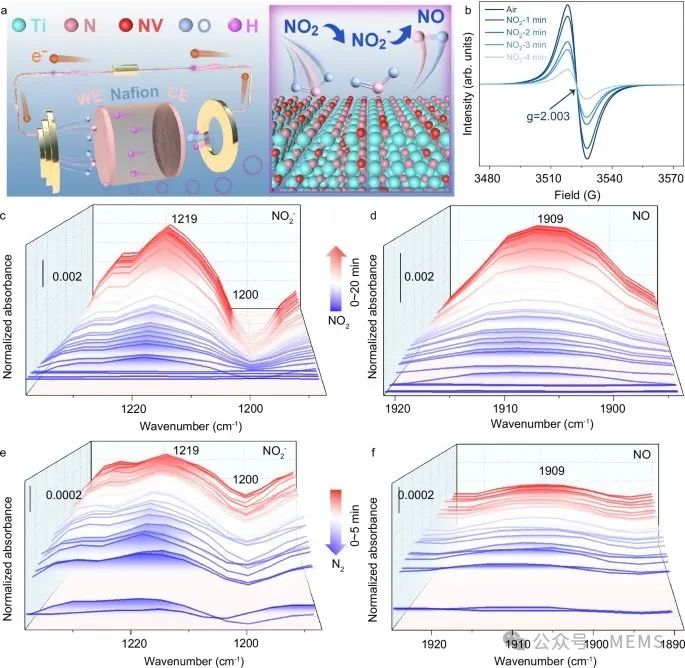 In situ characterization of the gas sensing mechanism of TiNx-based NO₂ sensor
In situ characterization of the gas sensing mechanism of TiNx-based NO₂ sensor
In conclusion, the researchers proposed a TiNx-based gas sensor synthesized through ammonia decomposition of titanium cluster MOF MIL-125, featuring an ultra-high specific surface area (221.9 m²/g) and abundant nitrogen vacancies. This TiN-600 sensor exhibits excellent selectivity for NO₂, with a response of 6.05 μA at 50 ppm, outperforming other interfering gases, with a signal 30 times that of NO. It also demonstrates reliable repeatability and long-term stability, showing a minimal sensitivity drift of 4.2% over six months. Compared to Pt-based sensors, the developed TiNx electrochemical gas sensor is more cost-effective. Utilizing in situ FTIR and EPR, the researchers elucidated the potential NO₂ sensing mechanism. This work not only advances the development of high-performance gas sensing materials but also provides directions for optimizing electrochemical gas sensors for practical environmental monitoring and industrial applications.
Original link:https://www.nature.com/articles/s41467-024-55534-x
Disclaimer:
This platform only collects and shares content, with copyrights belonging to the original publishing institution. All content is obtained through publicly available legal channels and is used solely for industry communication and learning, with no commercial interests. If any infringement is involved, please contact us for deletion; if there are doubts about the content, please contact the writing or publishing institution.