
Cancer therapeutics need to achieve a balance between efficacy and safety, even targeted chemotherapy does not always only kill abnormal cancer cells, non-specific toxicity can lead to a low therapeutic index and side effects.
The technology of proteolysis-targeting chimeras (PROTACs), which achieves therapeutic effects by promoting the degradation of target proteins, is rapidly transforming the fields of biology and medicinal chemistry; however, the physicochemical properties of PROTACs are also associated with poor DMPK characteristics and low bioavailability.
As an alternative means of delivering chimeric degraders effectively in vivo, degrader-antibody conjugates (DACs) have emerged, which have several potential advantages over the in vivo administration of PROTAC molecules:
(1) Ability to deliver chimeric degraders with poor physicochemical or DMPK properties in vivo (especially those using E3 ligases other than CRBN);
(2) Avoidance of complex formulation;
(3) Ability to target specific tumors or tissues with DACs that carry PROTAC molecules of interest.
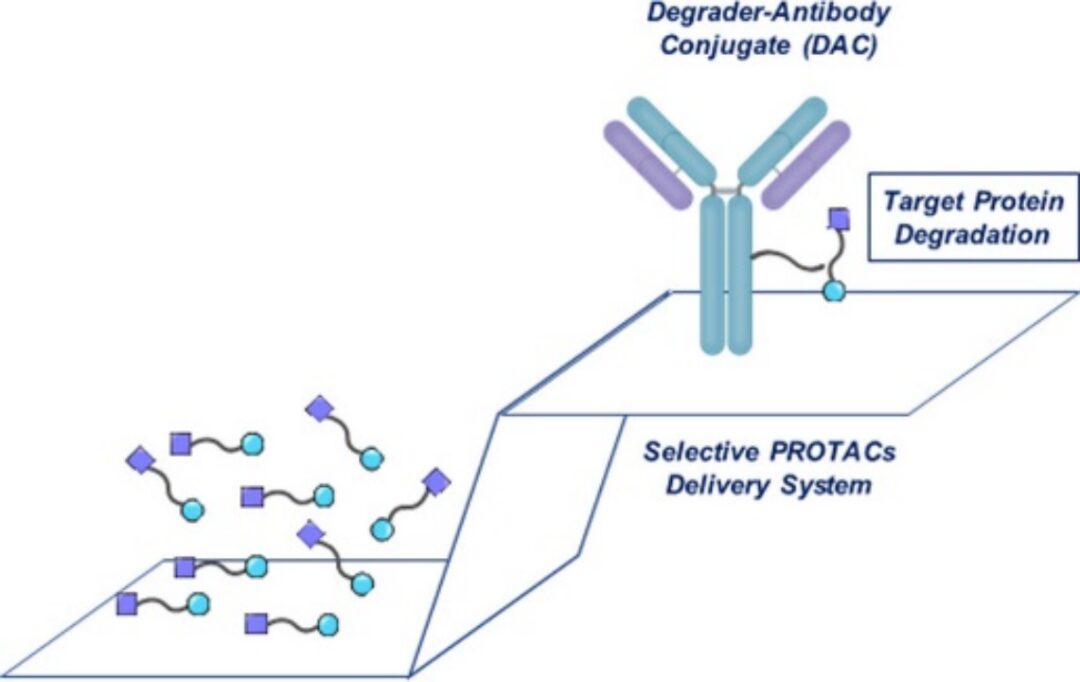

Composition and Mechanism of DAC
DAC consists of mAb, linker, and PROTAC payload components.
In principle, the structural composition of ADCs and DACs is similar, with the difference being that typically, monofunctional small molecules are used for ADCs, and PROTACs are used for DACs. The antibody is a key component for specifically binding and delivering payloads (such as small molecule toxins and PROTACs) to manipulate (or kill) and degrade target proteins. The prerequisites for antibodies are (1) specific interactions with clear antigens that have high tumor expression and limited normal cell expression; (2) the ability to maintain their characteristics, such as stability, internalization capacity, and binding with linkers and payloads; (3) high binding specificity for the targeted antigen. Linkers are divided into two main types: the first is cleavable linkers, which have three types of release mechanisms: lysosomal protease-sensitive (peptide), acid-sensitive (hydrazone), and glutathione-sensitive (disulfide) linkers; the second is non-cleavable linkers (such as thioethers), which show better stability in circulation compared to cleavable linkers. DAC payloads are designed for PROTACs and molecular glues. DAC requires a drug-to-antibody ratio (DAR) greater than 4, which may affect the conjugation and pharmacokinetics of DAC.
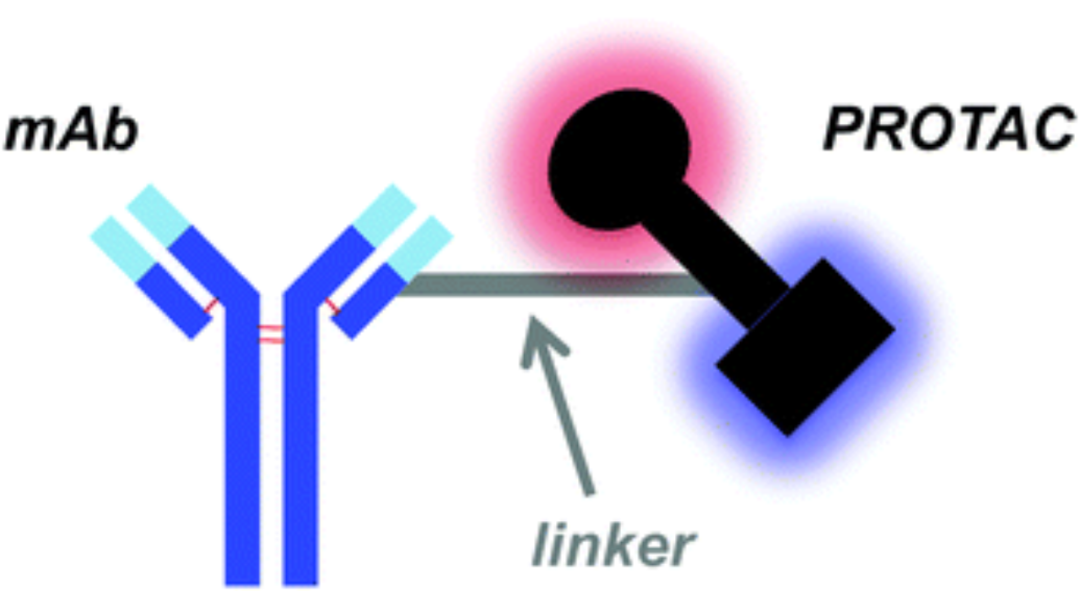 Figure Caption: DAC Composition
Figure Caption: DAC CompositionThe Mechanisms of DAC and ADC are Essentially the Same. After administration, the overall DAC should remain as stable as possible in circulation to prevent premature release of PROTAC and blood circulation. Then, the antibody portion of the DAC recognizes tumor-associated antigens on the cell surface. The DAC-antibody complex is internalized via receptor-mediated endocytosis. Inside the cell, the complex fuses with endosomes and is transported to activated lysosomes. In the proteolytic and acidic environment, the conjugated linker is degraded, and the payload (PROTAC) is subsequently released into the cytoplasm. Depending on the intracellular target of DAC, TPD can be induced.


Targeting BRD4 with DAC
At the beginning of 2020, Nature published the earliest example of DAC: a new disulfide-containing cleavable linker conjugated a VHL-based efficient degrader of bromodomain-containing protein 4 (BRD4) (GNE-987) to an antibody targeting CLL1 (DAC 1).
The optimized DAC 2 exhibited strong dose-dependent in vivo efficacy in HL-60 and EOL-1 acute myeloid leukemia (AML) xenograft models after a single intravenous administration, and the experimental results first demonstrated that degrader-antibody conjugates can overcome poor PROTAC PK characteristics, achieve acceptable PROTAC lysosomal stability, and confer appropriate antigen-based targeting, while also demonstrating that the antibody linker is appropriately cleaved from the hydroxyl of the PROTAC’s VHL binding fragment, allowing the released payload to have unobstructed bioactivity. The specific mechanism is that the antibody portion of DAC 2 binds to the CLL1 receptor on the surface of targeted tumor cells, the conjugate is then internalized and transported to lysosomes, where in this proteolytic environment, the antibody is metabolically degraded into its amino acid components, leaving cysteine residues partially attached to the remaining part of the linker through disulfide bonds. The disulfide is then reduced to yield the corresponding thiol, which undergoes self-immolation to release degrader 1.After continuously improving the conjugate with this idea, the BRD4-targeting DAC can target not only CLL1 but also STEAP1, and subsequent reports have described the development of HER2-dependent BRD4 degrading DAC.


Targeting Erα with DAC
The DAC targeting Erα degrades estrogen receptor alpha (ERα) nuclear transcription factor by forming a ternary complex with the apoptotic protein X-linked inhibitor of apoptosis (XIAP) E3 ligase, using a Val-Cit cleavable linker and phenol-ether payload conjugated to a HER2-targeting mAb.


DAC Targeting TGFβR2 and BRM
Using lysine as a linker, the non-cleavable amide-linked CRBN-linked DAC 18 showed the required level of TGFβR2 degradation after 24 and 48 hours.
The second example is the BRM protein degrader, BRM protein, also known as SMARCA2 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin subfamily A member 2), which is present in various human diseases, including cancer. This degrader was initially developed by Arvinas and Genentech in 2019. Genentech further demonstrated DAC 20, where mAb used a disulfide-based linker to bind to the CD22 cell surface receptor, introducing various linker chemistries, with xenograft data indicating effective antigen-dependent degradation of BRM protein levels.
 Figure Caption: DAC targeting TGFβR2 and BRM
Figure Caption: DAC targeting TGFβR2 and BRM
DAC Targeting GSPT1
ORM-5029 is a DAC molecule targeting GSPT1 protein currently in clinical phase I by Orum Therapeutics. In 2022 AACR, Orum Therapeutics publicly released preclinical data for ORM-5029, showing efficacy both in vitro and in vivo. Their payload degrader exhibited high selectivity for GSPT1 and was compatible with various linkers and conjugation techniques. In the HCC1569 breast cancer model, ORM-5029 at a single dose of 3 mg/kg showed strong potential for sustained tumor growth inhibition, and GSPT1 also showed a significant dose-dependent reduction.
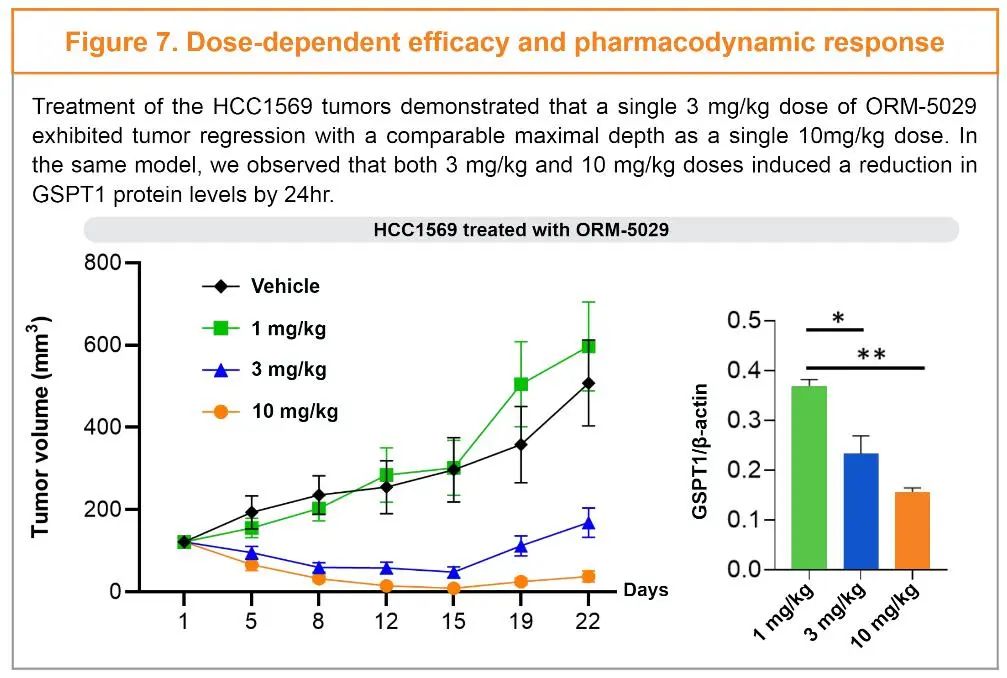 Figure Caption: Preclinical Data of ORM-5029
Figure Caption: Preclinical Data of ORM-5029

Considerations for DAC Structural Design
Although some strategies used in ADCs can be applied to the preparation and delivery of bioactive DACs, the construction of DACs also presents additional challenges. Compared to the broad cytotoxic payloads used for ADCs, degraders typically exhibit more targeted bioactivity with respect to specific cancer or tissue cell types. Therefore, the antigens of DACs must not only meet the internalization and transport standards of ADCs but should also be highly expressed on tumors, tissues, or other cells sensitive to degraders.
Since chimeric degraders are somewhat weaker compared to cytotoxic ADC payloads, a higher loading may be required to achieve similar efficacy (i.e., a DAR value greater than 4). Due to their chimeric nature, DAC PROTAC payloads are often larger or more lipophilic than cytotoxic molecules of ADCs (especially cell-permeable molecules). When PROTACs are attached to antibodies, these differences amplify aggregation and pharmacokinetic issues and may require new linker designs and conjugation methods, rather than those used in the ADC field.
Moreover, many reported chimeric degraders lack chemical groups that can be covalently linked for cleavable linkers. In such cases, it must be carefully considered whether to deliberately incorporate the necessary chemical groups into the degrader structure or to utilize new ADC linking methods to take advantage of existing functional groups of the degraders, such as hydroxyl/phenolic groups.
Other challenges that may need to be addressed in DAC design include: (1) Good stability of DAC payloads in lysosomal environments, (2) Ability of the payloads to effectively escape lysosomal compartments, (3) Tendency of the payloads to produce bystander effects. The latter two properties may be affected by the cell permeability of the degraders themselves, and optimizing this property is a current goal of PROTAC research.

DAC Development Still Carries Risks
Dosage Overload. One theoretical advantage of PROTACs is the low dosage, as PROTAC-driven degradation is “event-driven” rather than “occupancy-driven”: only transient target binding is required. After binding and ubiquitin transfer, the drug dissociates and enzymatically moves to the next target. In preclinical studies, very low PROTAC dosages can significantly reduce protein levels. Arvinas’ phase II drug is orally bioavailable, but the phase II dosage for its androgen receptor degrader ARV-110 was 420 mg, while the dosage for the company’s estrogen receptor degrader was 200 and 500 mg. Due to its low permeability and solubility, dosage overload has emerged. Arvinas reported sufficient dose-dependent plasma drug concentrations, with both drugs showing good safety in phase I, as well as anti-tumor activity. However, high dosages mean higher manufacturing costs, and PROTACs already have high manufacturing costs due to their multi-step synthesis.
Potential Side Effects. Toxicology is another bottleneck in PROTAC development, with the potential for both targeted and off-target side effects. The issue lies with the E3 ligase end, if the E3 docks in a manner similar to IMiD, PROTACs can degrade unexpected substrates, posing unforeseen risks.
Limitations of Molecular Glues. In light of the dosage and oral availability challenges of PROTACs, some companies are concentrating on another type of degrader, namely molecular glues, which are “monovalent” small molecules that only bind to E3 ligases, rather than bivalent PROTACs that bind both ligases and targets simultaneously. Celgene’s lenalidomide is a typical example. However, the downside of molecular glues is that they are difficult to target for selected disease-related targets for degradation; their discovery must be reverse-engineered by screening E3-binding molecules to achieve the desired phenotype or screening large protein combinations and then figuring out which proteins they are degrading.
Limited Scope of Degradation. PROTACs are also limited in the library of proteins they can degrade. There are not many examples of PROTACs degrading membrane proteins, and transmembrane proteins may not be the right targets for PROTACs. Degradation of proteins with expanded repeat sequences and aggregating proteins also poses challenges. For 15 years, it has been known that autophagy can degrade easily aggregating proteins and is more effective than the ubiquitin-proteasome system, which is a group of targets unsuitable for proteasomal degradation. Casma is developing degraders to recruit lysosomal and autophagic degradation mechanisms, including lysosome-targeting chimeras (LYTACs), the former linking targeting antibodies with glycans that trigger lysosomal degradation, and the latter linking target ligands with autophagy degradation tags.

Future Development of DAC
DACs are still in their infancy, but DAC entities have been produced, and PROTACs involving several different E3 ligases are being used for DACs, along with many different novel linker and mAb conjugation methods. Compared to most known cytotoxic ADCs (DAR = 2~4), DACs mostly adopt higher payloads (with DAR values of 6), but whether the actual application of DACs requires higher DAR values remains to be determined.

Figure Caption: DACs currently under development and their compositions


Jinkai Biological “20cm” Limit Up, Semaglutide Oral Version Drives Weight Loss Drug Concept Surge!

Global CDMO Giant Lonza to Close Guangzhou Factory, Lay Off 300 Employees. The Good News is CDMO Companies’ Sales are Growing!