 Continuing from the previous article, this section will further introduce the types of free radicals.The Ohio State UniversityNagibresearch team summarized the polarities ofnitrogen-substituted carbon-centered free radicals, aryl free radicals, and aromatic compounds with multiple heteroatom substitutions, as well as nitrogen, oxygen, and sulfur-substituted aryl free radicals.The polarity of nitrogen-substituted carbon-centered free radicals is shown in Table 3. Due to the increased electron-donating ability of the nitrogen lone pair (compared to the electronegative oxygen), α-amino (red) free radicals are more nucleophilic than α-oxygen variants. In fact, they are the most nucleophilic among all carbon-centered free radicals studied by the Nagib research team. Amines show a reduced inductive effect for distal (β, γ) amine substitutions (blue). Similarly, α-cyano free radicals (green) are similar to α-carbonyl free radicals, while α-sulfonamide (purple), α-amide (orange), and α-imide (red) free radicals parallel their α-oxygen analogs.
Continuing from the previous article, this section will further introduce the types of free radicals.The Ohio State UniversityNagibresearch team summarized the polarities ofnitrogen-substituted carbon-centered free radicals, aryl free radicals, and aromatic compounds with multiple heteroatom substitutions, as well as nitrogen, oxygen, and sulfur-substituted aryl free radicals.The polarity of nitrogen-substituted carbon-centered free radicals is shown in Table 3. Due to the increased electron-donating ability of the nitrogen lone pair (compared to the electronegative oxygen), α-amino (red) free radicals are more nucleophilic than α-oxygen variants. In fact, they are the most nucleophilic among all carbon-centered free radicals studied by the Nagib research team. Amines show a reduced inductive effect for distal (β, γ) amine substitutions (blue). Similarly, α-cyano free radicals (green) are similar to α-carbonyl free radicals, while α-sulfonamide (purple), α-amide (orange), and α-imide (red) free radicals parallel their α-oxygen analogs.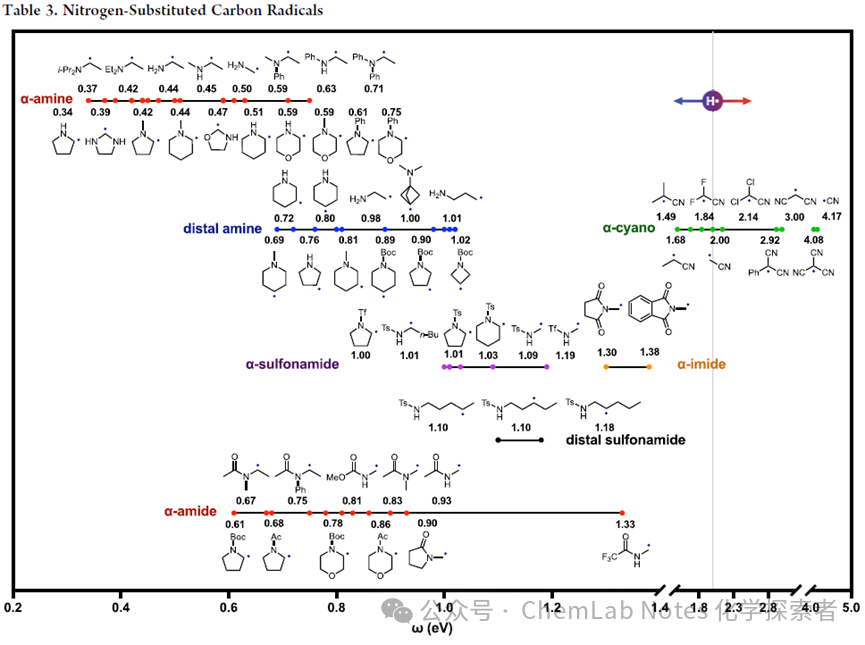
From the table, the following patterns can be derived:
1.Amines: Similar to alcohols and ethers containing α-OH and α-OR free radicals, α-NH2, α-NHR, and α-NR2 free radicals exhibit similar high nucleophilicity (0.4 eV), which explains their high reactivity with electron-deficient alkenes. However, in heterocyclic structures, free radicals on rings with free amines (α, β, or γ) are more nucleophilic than N-alkyl (0.1 eV) or N-Boc (0.2 eV) analogs. Recently, the high nucleophilicity of α-amino free radicals has enabled the extraction of electrophilic halides via XAT in Wuxi.
2.Amides: Common protecting groups, such as carbamates, amides, and sulfonamides, sequentially reduce the nucleophilicity of α-amino free radicals:NH > NMe > NBoc > NAc > NTs ∼ NTf; as shown in the pyrrolidine series (0.3, 0.4, 0.6, 0.7, 1.0 eV; in different rings). Although sulfonamides have strong electron-withdrawing properties—even at distal β, γ, or δ positions (as in remote HAT pathways)—they are not as electrophilic as the carbonyls of amides, as shown in the protected amine series: α-NTs < α-NTf < α-succinimide < α-phthaloyl (1.1, 1.2, 1.3, 1.4 eV).
3.Distal Effects: The inductive electron-withdrawing effect of distal sulfonamides (black) explains why β (1.2 eV) is more electrophilic than γ or δ (1.1 eV). However, resonance electron-donating effects compete: α (1.0 eV).
4.Cyanides: The strong additive effect of α-cyano groups is particularly evident in the acetonitrile series, where electrophilicity increases: α-CN < α-dicyano < α-tricyano (2.0, 3.0, 4.1 eV).
Aryl free radical dataset is shown in Table4, where the influence of aryl position on polarity is much less than the electronic effects of substituents. Relative to unsubstituted phenyl free radicals (1.4 eV), free radicals with donor groups (NH2, tBu, OMe exhibit only slight decreases in electrophilicity (1.3−1.4 eV), and in some cases are even more electrophilic (OH, Ph, SH: 1.5 eV), regardless of substitution patterns. On the other hand, the electrophilicity of acceptor groups ranges from slight (halogens, acyl: 1.6 eV) to significant (CF3, CN, NO2: 1.7−2.0 eV) increase.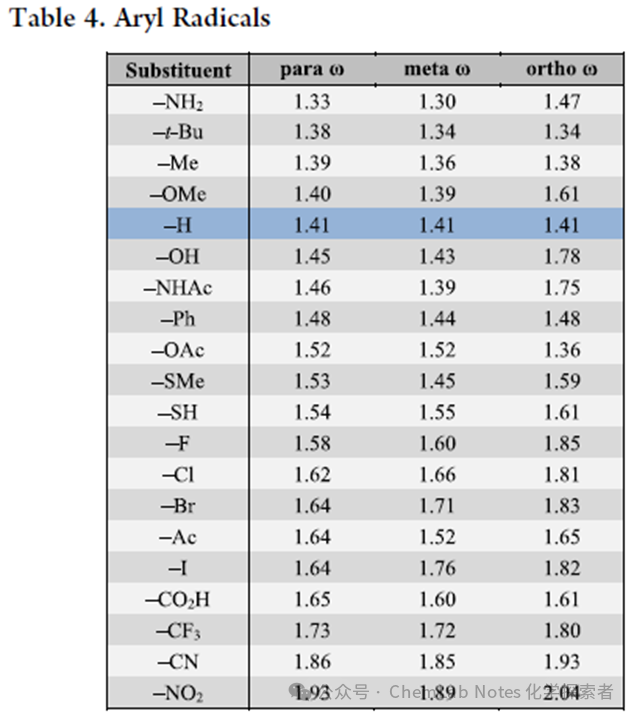 From the table, the following patterns can be derived:
From the table, the following patterns can be derived:
1.Phenyl:Phenyl free radicals (1.4 eV) are more electrophilic than methyl (1.2 eV), allyl/benzyl (1.2 eV) or vinyl (1.3 eV) free radicals, but still less electrophilic than para-CF3 benzyl free radicals (1.7 eV), which are similar to CF3-aryl free radicals (1.7−1.8 eV). This electrophilicity, along with the strong aryl C−H bond, explains why aryl free radicals are the most suitable for achieving C center free radicals through HAT functionalization.
2.Substitution Effects:Ortho substituents typically have a stronger influence on polarity than meta or para, as seen with p-Cl (1.6 eV), m-Cl (1.7 eV), and o-Cl (1.8 eV).
3.HammettConstants:The Hammett constants (σp and σm) compared to these electrophilicity values again show a strong trend, with correlation coefficients of R² = 0.85 (para) and R² = 0.86 (meta).
Heteroatom-substituted aromatics, as well as nitrogen, oxygen, and sulfur-substituted aryl free radicals are shown in Table5. While the electrophilicity of these free radicals is similar to that of the aryl set, there are some guiding trends and outliers. For example, trimethyl and monomethyl free radicals are similar (1.3−1.4 eV), but as the number of OMe substitutions increases, electrophilicity increases (mono-substituted < di-substituted < tri-substituted:1.4, 1.6, 1.8 eV), demonstrating an additive inductive effect. Other electron-withdrawing groups also show similar increases in electrophilicity, such as CF3 (mono-substituted < di-substituted:1.7, 2.1 eV) and F (mono-substituted < di-substituted < penta-substituted:1.6, 1.8, 3.1 eV). Notably, the C6F5 free radical is more electrophilic than the bisCF3 aryl, providing opportunities for selective utilization of these intermediates in organic synthesis.
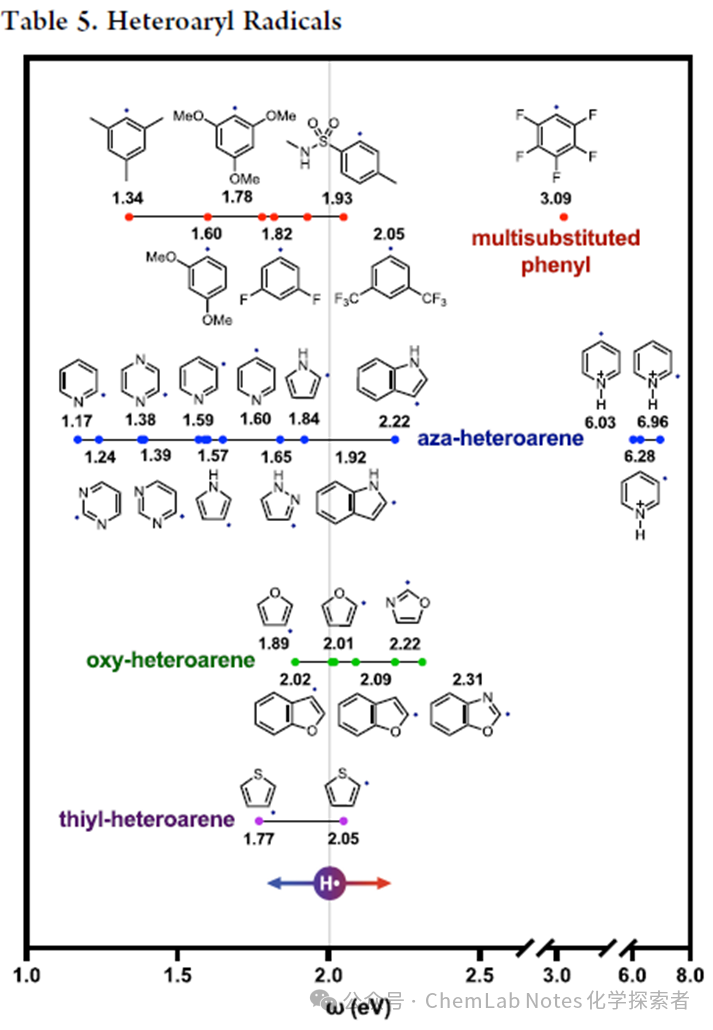
Significant observations regarding heteroaryl free radicals include:
1.Heteroatoms:Nitrogen-substituted aryl free radicals (blue), such as 2-pyridine, 2-pyrimidine, and 2-pyrazine (<1.4 eV), are more nucleophilic than phenyl free radicals (1.4 eV), while oxygen and sulfur-substituted aryl free radicals (green/purple) are more electrophilic (1.8−2.3 eV). Five-membered heteroaryl rings (>1.8 eV) are also more electrophilic than six-membered heteroaryl rings (<1.8 eV).
2.Nucleophilicity:2-pyridyl (1.2 eV) free radicals are the most nucleophilic among all measured aryl free radicals (even stronger than aniline), explaining how they are used as nucleophiles in Giese additions to acrylates.
3.Radical Cations:Conversely, through acid protonation, nucleophilic 2-pyridyl free radicals (1.2 eV) become highly electrophilic (7.0 eV), explaining their application in Kharasch additions to electron-rich alkenes.
The article is quite lengthy, and we will continue to discuss different types of free radicals in detail over the next few days. Interested readers can like, follow, and bookmark.
References:
https://doi.org/10.1021/jacs.4c06774
J. Am. Chem. Soc. 2024, 146, 28034−28059
Note: All images in this article are sourced from the reference. If there are any copyright issues, please contact the author promptly for proper handling. If you have any questions about this literature, feel free to communicate and discuss with the editor.