The five-year survival rate for heart failure is comparable to that of breast cancer and colorectal cancer. End-stage patients not only suffer from a significantly reduced quality of life but also impose a substantial economic and psychological burden on their families. Despite decades of research and development leading to the introduction of heart failure medications that have greatly improved patient survival rates and quality of life, the mortality rate at the end stage remains alarmingly high.
Evidence-based medicine indicates that medications and other methods aimed at improving the end-diastolic and end-systolic ventricular volumes are crucial for treating heart failure. Surgical interventions that improve ventricular volume in patients with dilated cardiomyopathy can enhance patient outcomes. However, the STICH study results suggest that coronary artery bypass surgery combined with left ventricular reduction surgery yields outcomes similar to those of patients receiving optimized medical therapy, with 15% of patients experiencing malignant ventricular arrhythmias due to postoperative cardiac scarring. Furthermore, the skill level of the surgeon is a significant factor influencing prognosis, and the surgery itself carries certain risks. Therefore, while left ventricular reduction can improve the prognosis of heart failure patients, it also raises higher demands for lower risk, ease of operation, and minimal sequelae.

On October 9, 2013, a team led by Professor Huo Yong from Peking University First Hospital successfully completed the first two cases of percutaneous left ventricular reconstruction (PVR) in China. This technique involves the release of a left ventricular isolation device via catheter technology to achieve the goal of treating heart failure. In September 2014, clinical trials for percutaneous left ventricular reduction surgery began in China, enrolling 31 patients with a success rate of 30 out of 31. The sub-center at Tongji University Affiliated 10th People’s Hospital enrolled 6 cases, all of which were successful.

The ventricular isolation device (VPD) – Parachute™ was developed by Cardiokinetix, based in Redwood City, California. The target population for this device includes patients with reduced apical motion or ventricular wall aneurysm following anterior descending artery myocardial infarction. The device is implanted via a percutaneous catheter, resembling a parachute, to reduce ventricular volume and restore normal ventricular geometry, interrupting Laplace’s law and improving cardiac function. Currently, the safety and efficacy of this device have been certified by the European Union (CE).

The three-year follow-up results of the first 31 patients using the Parachute were published in the journal Circulation in 2014.
Clinical trial inclusion criteria: (1) Abnormal apical motion after anterior wall myocardial infarction; (2) Age between 18 and 75 years; (3) Body Mass Index (BMI) < 40; (4) Consent to sign informed consent and comply with follow-up treatment; (5) Left ventricular dysfunction post-myocardial infarction demonstrated by echocardiography; (6) Female: postmenopausal, surgically sterilized, negative pregnancy test; (7) Symptomatic ischemic heart failure (NYHA II-IV), more than 60 days since the last myocardial infarction; (8) Received appropriate heart failure treatment according to ACC/AHA guidelines.
Main exclusion criteria include: (1) Abnormal wall motion outside the area covered by myocardial infarction; (2) Valve stenosis or regurgitation (tricuspid, aortic, mitral); (3) Cerebrovascular events (CVA) or transient ischemic attacks (TIA) within the last six months; (4) End-stage renal disease requiring long-term dialysis, progressive sepsis, active endocarditis; (5) Less than one year of expected survival or hospitalized at the time of enrollment; (6) Allergies to aspirin, heparin, warfarin, contrast agents, nickel-titanium alloy, making premedication impossible. After preliminary screening with echocardiography, all patients must undergo CT/CMR (completed by a core laboratory) to determine their suitability for surgery (specific screening methods are detailed in the case).
CT inclusion criteria include: (1) Ejection fraction < 40% combined with abnormal apical motion or ventricular wall aneurysm; (2) Left ventricular diameter 55-77 mm; (3) Left ventricular wall thickness > 3.5 mm; (4) Left ventricular apical diameter > 4×5 cm; (5) Normal trabeculae and papillary muscles.
CT exclusion criteria include: (1) Left ventricular > 40% combined with global enlargement; (2) Left ventricular diameter < 55 or > 77 mm; (3) Left ventricular wall thickness < 3.5 mm; (4) Left ventricular apical diameter < 4×5 cm; (5) Excessive trabecular formation in the left ventricle, deep tissue of papillary muscles, or ventricular muscle bands obstructing the normal deployment of the device.

◆ ◆ ◆ ◆ ◆ ◆
This article presents a comprehensive introduction to the selection and operation of the left ventricular remodeling device through a typical case from our center.
General Information
The patient, a 52-year-old male, experienced an acute anterior wall myocardial infarction on March 3, 2014, and underwent emergency stenting of one lesion in the anterior descending artery. There were no significant past medical history. After PCI, the patient repeatedly experienced chest pain, tightness, and shortness of breath. Echocardiography indicated that since March, the left ventricular ejection fraction (LVEF) had decreased monthly from 50% to 28%, accompanied by the formation of a left ventricular wall aneurysm.
In December 2014, the patient was admitted for planned PVR surgery, with a diagnosis of coronary atherosclerotic heart disease, old anterior wall myocardial infarction, post-PCI, and heart function classified as NYHA II. Preoperative echocardiography showed an LVEF of 28%, with mild mitral and tricuspid regurgitation. The 6-minute walk test distance was 625 m. The patient underwent percutaneous left ventricular reconstruction on December 4, 2014.
Left Ventricular Isolation Device
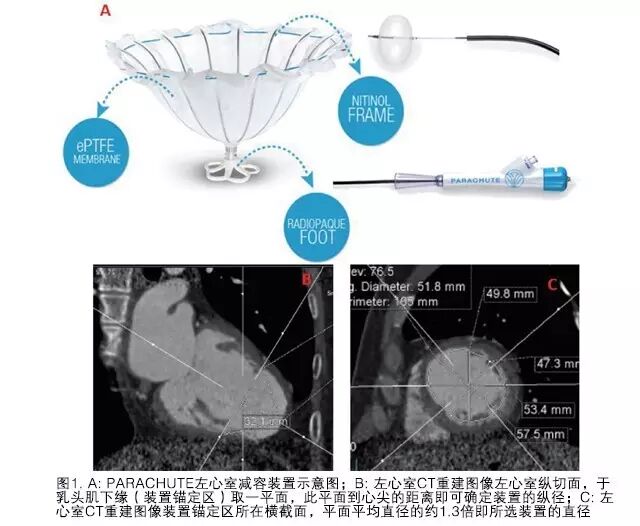
The left ventricular isolation system consists of three parts: the access system, delivery system, and isolation device. The access system is composed of a 14F or 16F guiding catheter and dilator, primarily aimed at establishing a pathway to the apex of the left ventricle. The delivery catheter provides a channel for the isolation device connected to a torque shaft to reach the apex, with a knob at the end of the torque shaft connected to the isolation device and balloon. The balloon can be filled with contrast agent for releasing and opening the device, and there is a release knob at the proximal end of the catheter. The isolation device resembles a parachute, with a self-expanding nickel-titanium alloy framework consisting of 16 struts, and a 2 mm long anchor at the end of the metal framework to secure the device and prevent displacement after release. The framework is covered with a layer of polytetrafluoroethylene membrane, and the end in contact with the apex has a Pebax polymer foot. Currently, there are eight specifications of left ventricular isolation devices, with maximum diameters of 65, 75, 85, and 95 mm after full expansion, each diameter further divided into long (standard) and short (short) legs based on the long axis of the device. The model selected for this patient was 85 mm-short, with specific selection criteria detailed below.
Device Selection
The selection of the device is completed through CT, which is a crucial step. The CT reconstruction image of the left ventricle takes the longitudinal section of the left ventricle, with the average diameter of the cross-section at the anchoring area of the device being 52 mm. Considering the compression ratio, the selected device diameter is approximately 1.3 times this result, hence 85 mm was chosen. A plane was taken at the lower edge of the papillary muscle (anchoring area), and the distance from this plane to the apex determines the longitudinal dimension of the device, which for this patient was 32 mm; thus, the final device selection was 75 mm-standard, with the selection method and schematic diagram (see Figure 1).
Perioperative Medication
The patient was given 300 mg/d of aspirin orally for three consecutive days before surgery; during the procedure, heparin was administered at 100 U/kg for anticoagulation; postoperatively, warfarin was combined with aspirin at 100 mg/d for at least 12 months, with the International Normalized Ratio (INR) reaching 2.5. Anticoagulation treatment was maintained until the INR reached 2.0-3.0 before stopping low molecular weight heparin.
Surgical Procedure
The procedure was performed under local anesthesia. Bilateral femoral arteries were punctured, with a 6F sheath placed on the left side and an 8F sheath on the right side. A 0.035 in (1 in = 0.0254 m) guidewire was exchanged, and a 16F sheath was placed in the right femoral artery along the guidewire, followed by the insertion of a pig-tail catheter into the left ventricle, after which the pig-tail catheter was withdrawn, leaving the guidewire in place. A 6F pigtail catheter was placed into the apex of the left ventricle from the left femoral artery, and left ventricular angiography was performed to observe the left ventricular structure and confirm suitability for the placement of the ventricular isolation device. The ventricular isolation device Parachute was installed in the delivery catheter in an external water tank, ensuring complete air removal. The delivery catheter was advanced to the apex of the left ventricle along the retained guidewire, and the guidewire was withdrawn. The isolation device was then placed at the apex via the delivery catheter, and left ventricular angiography and transthoracic echocardiography were used to confirm that the isolation device was in position. The delivery catheter was fixed, the guiding catheter was withdrawn, and the balloon was inflated. Upon inflation, the isolation device fully expanded, the knob was rotated, and the device was separated from the delivery system. After securing the isolation device, the pushing sheath was withdrawn. Left ventricular angiography and transthoracic echocardiography were rechecked, confirming no displacement or regurgitation, and the bilateral arteries were sutured to complete the surgery.
Effect of PVR
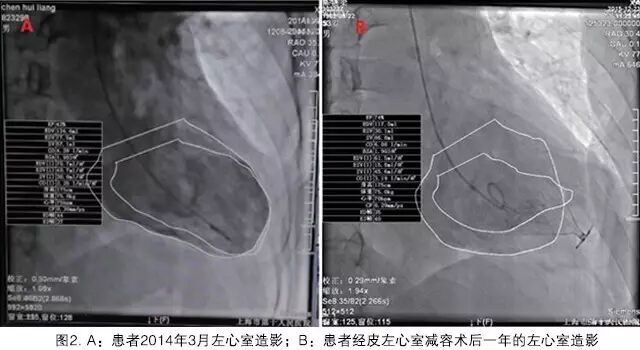
The patient recovered well postoperatively, with slight leakage from the wound one week after surgery, which healed well after treatment. A follow-up echocardiogram seven days post-surgery showed that the LVEF had already increased to 36%. One year post-surgery, cardiac color Doppler ultrasound indicated that the device was well anchored, with no displacement, and the left ventricular ejection fraction was 35%, with no regurgitation around the occlusion umbrella. On December 22, 2015, the patient underwent coronary angiography and left ventricular angiography, as shown in the figure (Figure 2). The left ventricular ejection fraction had significantly improved, and the contractility of the left ventricular myocardium, excluding the wall aneurysm, had markedly increased. The left ventricular cardiac output increased from 4.28 L/min to 6.08 L/min. The patient reported a significant improvement in chest tightness symptoms, occasionally feeling a sensation of a foreign body in the chest.
In this clinical trial, our hospital successfully performed PVR on 6 cases, all male, with an average age of (59±12) years, and all classified as NYHA II or higher. The average time from myocardial infarction to surgery was (9±7) months, with an average X-ray exposure time of (13.6±10.4) min. All patients experienced relief from heart failure symptoms, with 4 patients occasionally reporting a sensation of a foreign body in the chest. One 71-year-old patient, who had recurrent chest pain and tightness for two years post-PCI, experienced complete resolution of symptoms, significantly improving his quality of life. Follow-up results in December showed that all patients’ NYHA functional classifications returned to class I, with left ventricular EF increasing from 0.289±0.058 to 0.343±0.071, left ventricular end-diastolic diameter decreasing from (5.34±0.67) mm to (5.26±0.94) mm, and the 6-minute walk distance increasing from (494.0±103.9) m to (641.0±85.8) m.
The safety and efficacy of the Parachute™ have been validated through multiple countries and centers, including ongoing randomized controlled trials at 65 centers in Europe. During CIT 2016, Professor Huo Yong presented the one-year follow-up results of 30 enrolled patients in China, further validating the safety and efficacy of the Parachute left ventricular isolation system. This device is specifically designed for treating ischemic heart failure with poor left ventricular wall motion/ventricular wall aneurysm, isolating non-functional, non-contractile left ventricle to re-form a normal apical shape, restoring the left ventricle to its normal shape. The percutaneous left ventricular device can be released via catheter under local anesthesia, reducing surgical risks and avoiding myocardial scarring associated with surgery. The Parachute reduction device can improve cardiac function through multiple mechanisms: (1) Changes in apical shape and the nickel-titanium alloy framework can enhance cardiac contractility and maintain cardiac output (stroke volume); (2) By altering the geometric structure of the left ventricle, it reduces wall tension in the upper chambers; (3) The Parachute implant replaces rigid ventricular remodeling scar tissue, improving left ventricular diastolic function and reducing end-diastolic filling pressure.
As an effective treatment method to improve patient prognosis, left ventricular reduction aims to minimize patient trauma. In recent years, a series of methods have been developed, with five devices currently applied in humans: Acorn CorCap, Paracor HeartNet, Myocor Coapsys, BioVentrix Revivent, and Parachute, along with the biopolymer gel Algisyl-LVR. Among these, Parachute, Algisyl-LVR, BioVentrix Revivent, and Paracor HeartNet have received CE certification; except for Parachute, the other five methods require surgical intervention, but all are less invasive than traditional surgical procedures, with their safety validated through clinical trials.
As a minimally invasive left ventricular reduction method, Parachute represents a new breakthrough in the field of heart failure treatment, bringing hope and light to more end-stage heart failure patients who cannot undergo cardiac surgery.
Physician Profile

Chen WeiTongji University Affiliated 10th People’s Hospital
Chief Physician, Professor, Master’s Supervisor
Executive Committee Member of the Heart Failure Professional Committee of the Chinese Medical Association, Executive Committee Member of the Cardiac Critical Care Professional Committee of the Chinese Medical Association, Vice Chairman of the Shanghai Branch of the Cardiac Critical Care Expert Committee of the Chinese Medical Association, Member of the Left Atrial Appendage Occlusion Working Committee of the 6th Committee of the Chinese Society of Cardiology, Member of the Cardiovascular Professional Committee of the Cross-Strait Medical and Health Exchange Association, Member of the Cardiovascular Professional Committee of the Chinese Geriatric Health Association, Senior Member of the Chinese Society of Biomedical Engineering, Member of the 6th Committee of the Shanghai Society of Biomedical Engineering’s Electrophysiology and Pacing Professional Committee, Director of the Early Detection of Vascular Lesions Sub-center of the Ministry of Health’s Ten-Year Hundred Projects, Editorial Board Member of the Journal of Evidence-Based Cardiovascular Medicine, Reviewer for the Chinese Journal of Cardiovascular Diseases. He has received a first-class provincial and ministerial scientific and technological achievement award, participated in two National Natural Science Foundation projects, and published 25 articles in domestic core journals and international professional magazines, as well as edited one translated monograph.
END
This content is original from the “Outpatient” magazine, published in the June 2016 issue. Reproduction requires authorization and must indicate the source.
New Perspectives from Outpatient |WeChat ID: ClinicMZ

Official WeChat of “Outpatient” magazine
Long press, scan the QR code, and follow us