 【Research Background】
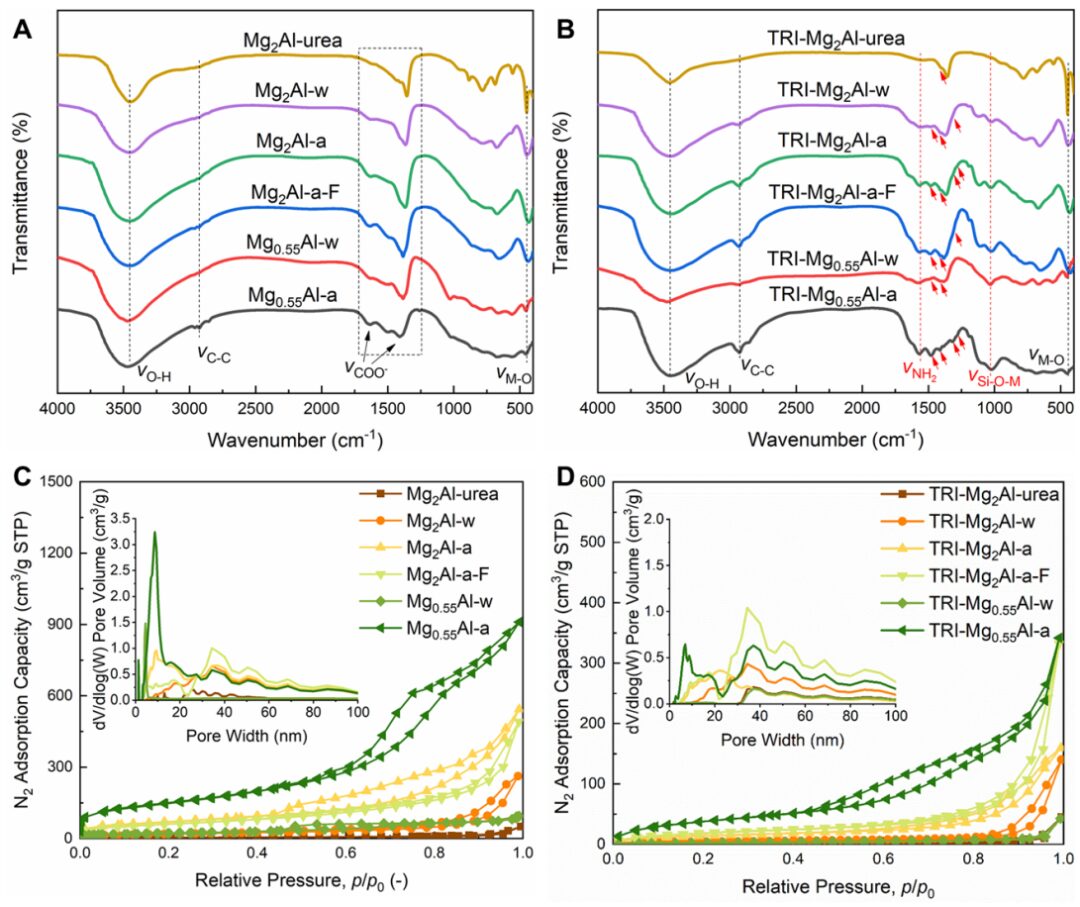
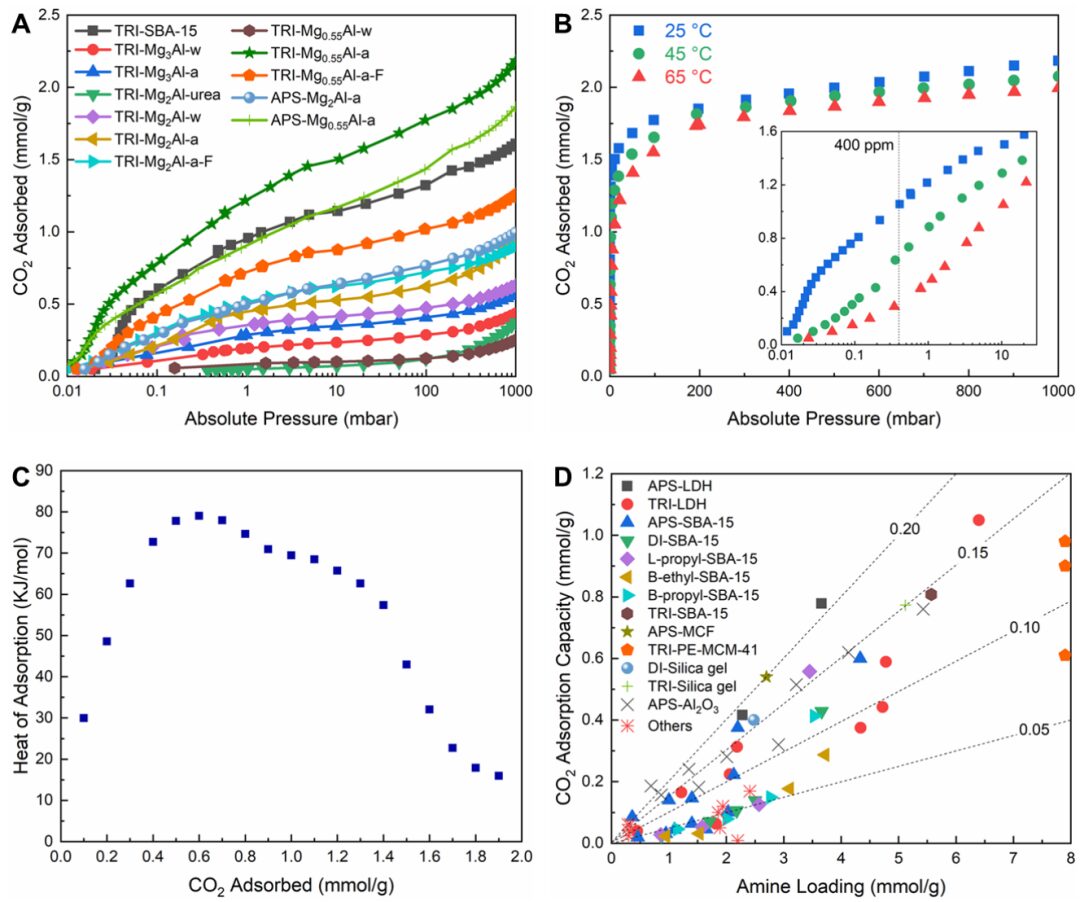
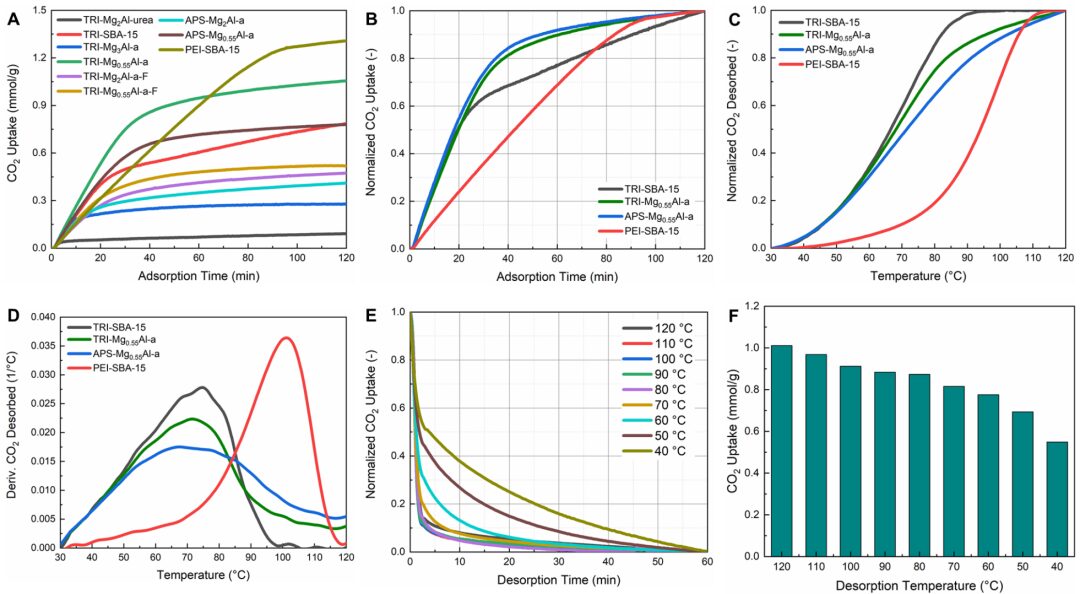
【Research Background】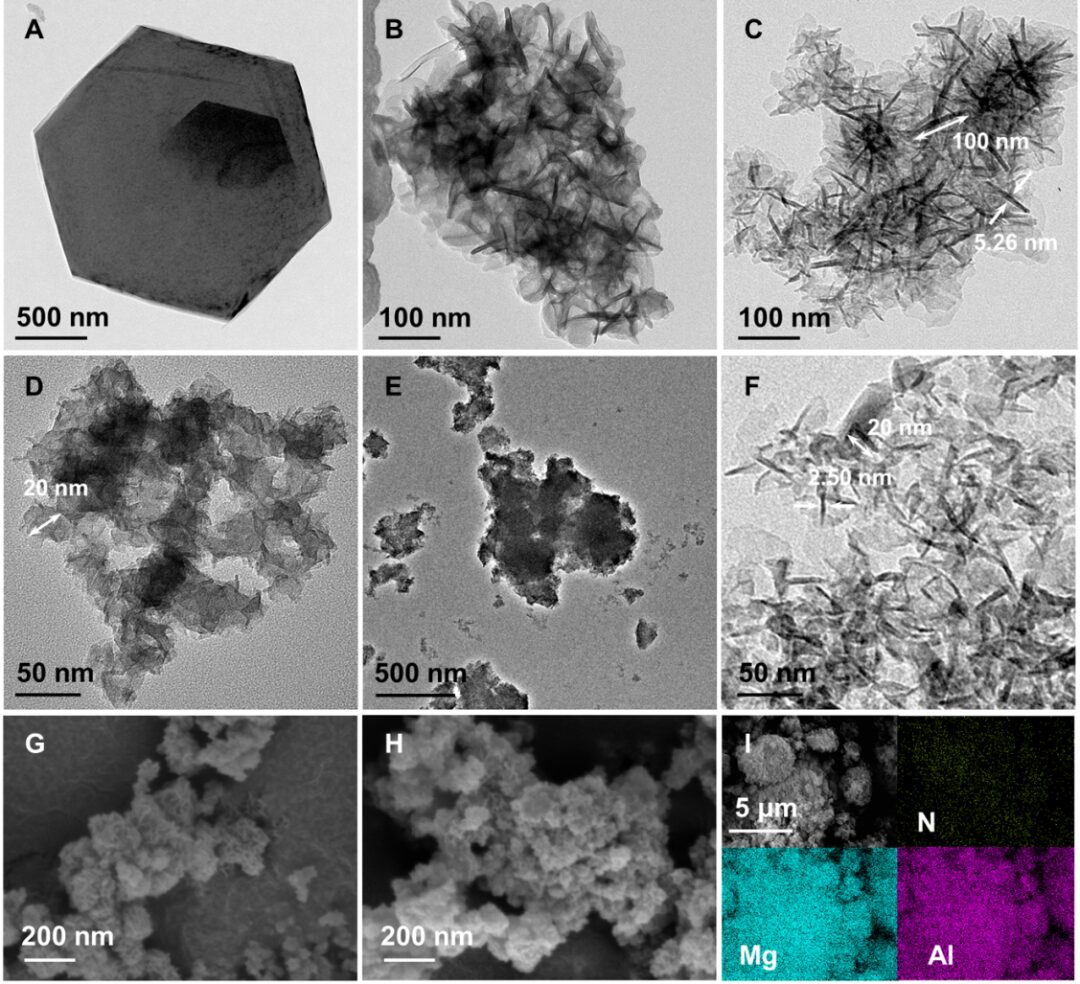
【Carbon Neutrality】CO2 conversion into key solvents for lithium batteries, the market in various fields is expected to reach nearly 10 billion in the future!
2021-07-10

Max Planck Institute in Germany: A major breakthrough in materials for hydrogen transmission and storage!
2021-07-10

Jilin Normal University Nano Energy: Electrostatic self-assembly behavior of MOFs colloidal particles and their derived core-shell carbon nanotube micro-cages for application in flexible zinc-air batteries
2021-07-10
The South Korean government has released a development strategy for the secondary battery industry, including solid-state and lithium-sulfur technologies!
2021-07-10

In just half a month, Zhejiang University has published its 7th article this year: This Science fiber is made from ice and can bend!
2021-07-09

Yu Guihua’s team: Adjustable porous electrode structure optimizes lithium-ion storage dynamics in thick electrodes
2021-07-09
Professor Yan Jun from Harbin Engineering University and Professor Zhang Qiang from Tsinghua University: Enhancing the lithium storage capacity and reaction kinetics of MoO3 by introducing oxygen vacancies and heterogeneous structures
2021-07-09
Academician Wu Feng’s team from Beijing University of Technology: Organic-inorganic dual protective interfacial layers enhance the stability of lithium metal anodes
2021-07-09
Jeff Dahn: Factors affecting the capacity of nickel-rich cathodes in low-pressure regions and new methods for measuring lithium diffusion
2021-07-08

Professor Wei Haijun: Latest research progress on interface engineering of electrolyte additives for lithium-rich cathodes
2021-07-08
