Expert Commentary


Professor Yuan Zhongyu
Department of Internal Medicine, Sun Yat-sen University Cancer Prevention and Treatment Center
Chief Physician, MD, Doctoral Supervisor
Engaged in clinical and research work on breast cancer for many years, has presided over and participated in over 20 clinical studies, involved in several research projects such as the National 863 Program and the National Natural Science Foundation, published over 50 SCI papers in JAMA, CCR, JNCI, Mol Oncol, etc.
Main Social Positions:
Deputy Chairman of the Breast Cancer Professional Committee, Guangdong Provincial Chest Cancer Association
Deputy Chairman of the Breast Cancer Professional Committee, Guangdong Provincial Health Management Association
Deputy Chairman of the Breast Disease Professional Committee, Guangdong Provincial Traditional Chinese Medicine Association
Deputy Chairman of the Expert Committee on Breast Medication, Guangdong Provincial Pharmaceutical Association
Deputy Chairman of the Breast Disease Prevention and Treatment Professional Committee, Guangdong Provincial Chest Disease Association
Executive Member of the Targeted and Personalized Treatment Professional Committee, Guangdong Provincial Anti-Cancer Association
ADC is a new hotspot in cancer drug development, consisting of monoclonal antibodies, linkers, and small molecule cytotoxic drugs. ADC exerts its anti-tumor effect by specifically binding to tumor cell surface antigens, being internalized into tumor cells, and releasing the conjugated cytotoxic drugs. Vadisizumab, as a targeted HER2 ADC drug independently developed in China, utilizes a novel antibody component, which has differentiated biological properties compared to other ADC drugs, offering better targeting, higher efficacy, and improved resistance.
At the 2021 American Society of Clinical Oncology (ASCO) conference, data from phase I and Ib studies of Vadisizumab for patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer were presented. The analysis showed that in the HER2-positive subgroup, the objective response rate (ORR) for the 2.5mg/kg dose group was 40.0%, with a median progression-free survival (PFS) of 6.3 months. The ORR and median PFS for the HER2-low expressing subgroup were 39.6% and 5.7 months, respectively. Vadisizumab demonstrated consistent efficacy in advanced HER2-positive and HER2-low expressing breast cancer, with good safety. Based on the excellent performance of previous studies, a comparative study of Vadisizumab and lapatinib combined with capecitabine for HER2-positive locally advanced or metastatic breast cancer patients previously treated with trastuzumab and taxanes is ongoing. This study indicates that in HER2-positive breast cancer patients with liver metastases, Vadisizumab significantly benefits, showing a higher efficacy (63.2% vs. 39.5%) compared to lapatinib combined with capecitabine, while also demonstrating good survival benefits, with a median PFS of over one year (12.5 months vs. 5.6 months). After communication with the Center for Drug Evaluation (CDE), the first phase of randomized controlled enrollment was stopped, and eligible patients could crossover to the experimental group, with the second phase of confirmatory seamless design phase III clinical study starting simultaneously. The final results of this study are awaited, which may rewrite the guidelines for late-line treatment of advanced HER2-positive breast cancer.

Author:Bi Xiwen, Sun Yat-sen University Cancer Prevention and Treatment Center

Professor Bi Xiwen
Department of Internal Medicine, Sun Yat-sen University Cancer Prevention and Treatment Center
Deputy Chief Physician, Master’s Supervisor
Youth Member of the Clinical Chemotherapy Professional Committee, Chinese Anti-Cancer Association
Youth Member of the Targeted and Personalized Treatment Professional Committee, Guangdong Provincial Anti-Cancer Association
Member of the Translation Group of the Clinical Oncology Professional Committee, Chinese Anti-Cancer Association

Case Introduction

1Basic Information:
Patient Data: Female, 43 years old
Case Provider: Sun Yat-sen University Cancer Prevention and Treatment Center, Bi Xiwen
Diagnosis: Advanced metastatic breast cancer
2Medical History Review:
2020-08 Follow-up revealed multiple lymph node metastases in the right axilla, supraclavicular region, mediastinum, and bilateral pulmonary hila, with multiple rib and right humerus metastases, and multiple lung metastases. Right axillary and supraclavicular lymph node biopsy: poorly differentiated adenocarcinoma, ER(-), PR(-), HER2(2+), Ki-67(70+). Molecular diagnosis: HER2-FISH(+), gBRCA no mutation detected.
3Treatment Process:
First-line Treatment: Pathological testing after recurrence indicated HER2 type, and the patient had not previously received anti-HER2 treatment. Administered docetaxel + trastuzumab + pertuzumab (HP) for 7 cycles. After 2 cycles, the efficacy was assessed as partial response (PR), stable disease (SD) with disease progression (PD) after 7 cycles. The patient suddenly experienced elevated transaminases, with CT revealing multiple new liver metastases and brain MRI indicating multiple brain metastases. First-line treatment progression-free survival (PFS) was 5 months.
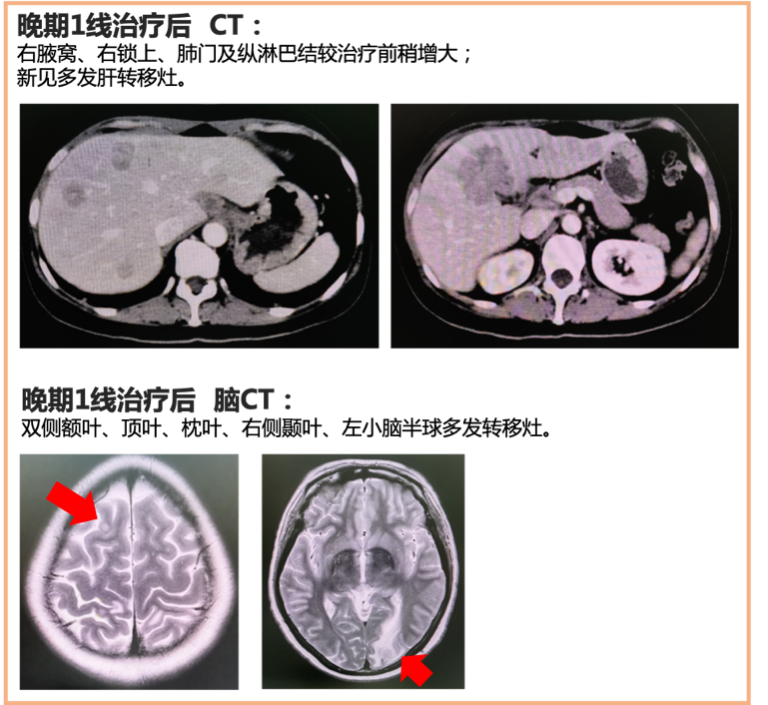
Second-line Treatment: Administered vinorelbine + capecitabine for 1 cycle, along with whole-brain radiotherapy. Efficacy evaluation: CT showed PD (liver lesions continued to enlarge), MRI showed reduced brain lesions. Second-line treatment PFS was 1.5 months.
Third-line Treatment: After first-line treatment, re-evaluated axillary lymph node biopsy for HER2-FISH, which was negative, indicating heterogeneity in HER2-FISH; the patient refused liver biopsy. Administered gemcitabine + pyrotinib + everolimus for 4 cycles. Efficacy evaluation: after 2 cycles SD (lesion reduction), after 4 cycles PD (liver lesions enlarged), intracranial lesions stable. Third-line treatment PFS was 3 months.
Fourth-line Treatment: Considering the patient’s HER2 heterogeneity, with previous HP and TKI treatments yielding poor results, administered UTD1 + capecitabine for 2 cycles. Efficacy evaluation: after 2 cycles PD (liver lesions enlarged), intracranial lesions stable. Fourth-line treatment PFS was 1.5 months.
Fifth-line Treatment: Vadisizumab (2mg/kg, Q2W) for 5 cycles. Efficacy evaluation: after 4 cycles PR, intracranial lesions SD (enlarged). During treatment, grade 2 leukopenia occurred, with no other adverse reactions.
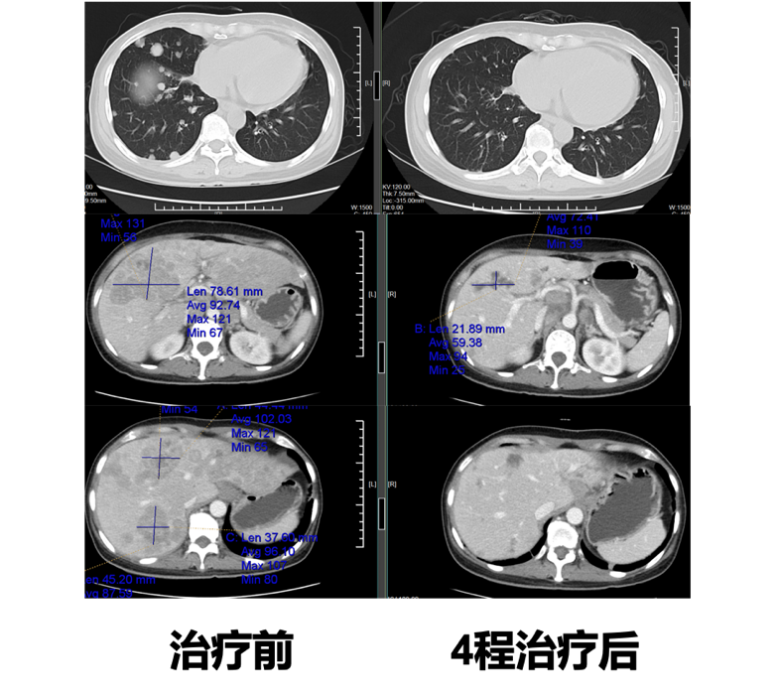
CT Comparison Before and After Vadisizumab Treatment
Changes in Tumor Markers Before and After Vadisizumab Treatment
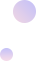
Case Summary

This case involves a middle-aged female patient with advanced breast cancer, initially diagnosed as stage III invasive ductal carcinoma, who underwent modified radical mastectomy followed by adjuvant therapy. One year later, follow-up revealed tumor metastasis, with pathology indicating a change in HER2 status from negative to positive post-surgery, and HER2-FISH also showing positive. Therefore, first-line treatment was administered with the THP regimen; however, tumor progression occurred after 5 months. A subsequent biopsy of the right axillary lymph nodes indicated HER2-FISH as negative, and re-testing by the molecular diagnostics department also confirmed HER2-FISH as negative, suggesting heterogeneity in HER2-FISH in the axillary lymph nodes. The patient then underwent multiple lines of treatment, including chemotherapy and TKI targeted therapy, all of which failed.
After the fourth-line treatment, the patient had a high tumor burden, and previous combinations of chemotherapy drugs with HP and TKI had poor efficacy, leading to challenges in subsequent treatment options. After comprehensive assessment and thorough communication with the patient and family, it was ultimately decided to administer the anti-HER2 antibody-drug conjugate (ADC) Vadisizumab. After 4 cycles of treatment, follow-up revealed rapid tumor shrinkage and significant decrease in tumor markers, achieving a PR efficacy evaluation.
(Note from the case provider: The patient has undergone multiple lines of treatment, and in the context of limited treatment options, the above regimen was provided after full communication with the patient. The purpose of sharing this case is to provide a diagnostic and treatment thought process for clinicians encountering similar refractory patients, for discussion.)