To improve the DMPK characteristics, many studies have explored the coupling of PROTAC payloads with monoclonal antibodies to create a new molecule known as “Antibody-Drug Conjugates (DAC).” This allows for more effective delivery of PROTAC degraders in vivo.
▲ Structure of DAC (Image Source: Reference 1)
Compared to PROTAC molecules, DAC has several potential advantages: it can deliver degraders with poor physicochemical properties or DMPK characteristics in vivo; it avoids complex formulations that are usually necessary for PROTACs to achieve activity in vivo; and it can target specific tumors or tissues with PROTAC molecules.
Stay updated with industry cutting-edge information
Get valuable resources in the field
Engage in in-depth discussions with industry experts
Scan the QR code below to join the group
If the group QR code expires, please add the editor’s QR code on the right to join the group.
Although DAC can address some shortcomings of PROTAC to a certain extent and has great application prospects, there are still many challenges in developing DAC due to the specificity of PROTAC molecules:
-
PROTAC in DAC typically only has targeted activity against specific tumors and/or tissues or cells. Therefore, the selection of antigens needs to meet the requirement for internalizing DAC and transporting it to lysosomes, as well as being highly expressed in the target tissue (or tumor, cell) of PROTAC.
-
Compared to small molecule drugs, the in vitro activity of PROTAC is usually weaker, so the drug-to-antibody ratio (DAR) in DAC must be increased to exert efficacy (DAC>4). However, increasing the DAR may lead to aggregation of DAC, adversely affecting in vivo pharmacokinetics (PK). Furthermore, PROTAC is larger and more lipophilic than small molecule drugs, which exacerbates aggregation and PK issues, necessitating the development of new linkers and conjugation methods to overcome these challenges.
-
Many PROTACs lack sites (amino groups) for covalent attachment to cleavable linkers. Therefore, it may be necessary to modify the PROTAC structure to introduce active sites (potentially altering the physiological activity of PROTAC); or utilize existing functional groups (such as hydroxyl, phenolic hydroxyl) in PROTAC to develop new conjugation technologies. On the other hand, developing non-cleavable linkers also requires similar considerations, and in such cases, care must be taken that the linker remaining on the PROTAC molecule after lysosomal degradation does not interfere with its biological activity.
Additionally, issues such as the stability of PROTAC in lysosomes, PROTAC lysosomal escape functionality, and the PROTAC bystander effect remain to be resolved.
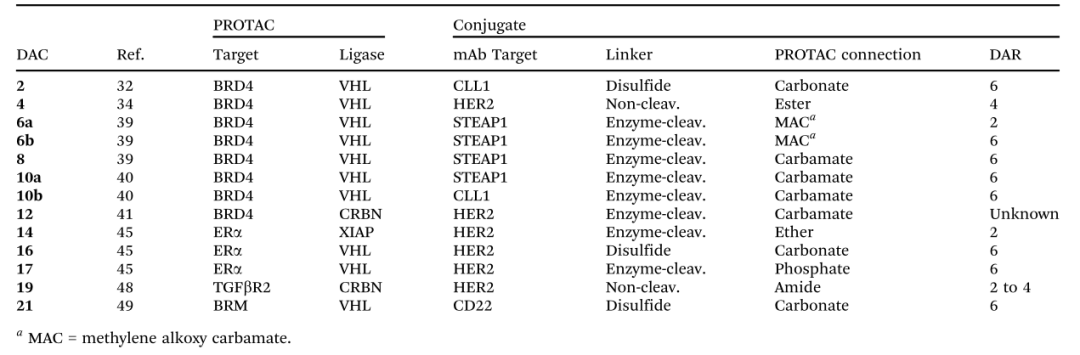
It is worth mentioning that although research on DAC is still in its infancy, several candidates with in vitro and/or in vivo biological activity have already been discovered.
▲ Reported DAC molecules (Image Source: Reference 1)

“Everything Can Be Coupled” Has Become a Major Trend
Conjugated drugs span multiple fields such as antibodies, small molecules, and cell therapies. With the emergence of commercial value and clinical advantages, this field is heating up rapidly. Numerous new concept products, such as peptide-drug conjugates (PDC), antibody-oligonucleotide conjugates (AOC), and virus-like drug conjugates (VDC), are gradually coming into the public eye.
➤ Peptide-Drug Conjugates (PDC)
PDC is considered another highly promising conjugate drug following ADC, consisting of a targeting peptide, a cytotoxic drug, and a linker.
PDCs have smaller drug molecular weights, and the synthesis and purification of targeting peptides are easier, resulting in lower production costs. Additionally, PDC drugs have strong tumor penetration and lower immunogenicity. Several companies in China have already laid out in this field and have achieved some positive results, including the PDC candidate SNG1005 developed by Shengnuo and Canadian company Angiochem for treating brain metastatic non-small cell lung cancer and breast cancer, which has now entered Phase 3 clinical trials.
▲ Image Source: Reference 2
➤ Antibody-Oligonucleotide Conjugates (AOC)
AOC mainly consists of targeting antibodies, linkers, and therapeutic oligonucleotides (siRNA, PMO, etc.). It can utilize antibodies to deliver oligonucleotides to specific cells or tissues, thereby reducing the required dosage and solving the delivery problem of targeted oligonucleotides. Compared to traditional oligonucleotide therapies, AOC has better pharmacokinetic characteristics and more specific biological distribution, avoiding the side effects caused by the use of lipid nanoparticles for delivery.
Currently, many companies have laid out in this field, including Tallac, Avidity, Dyne Therapeutics, Denali, and Gennao Bio. Among them, Avidity’s AOC1001 for treating Myotonic Dystrophy Type 1 (DM1) has entered Phase 1/2 clinical trials.
▲ AOC1001 (Image Source: Avidity Company Website)
➤ Virus-Like Drug Conjugates (VDC)
VDC is a form of conjugated drug that uses a non-infectious protein nanoparticle (VLP) designed from viral shells as an efficient delivery vehicle. Aura Biosciences is the first company in the VDC field, utilizing VLP derived from Human Papillomavirus (HPV) to selectively attach to modified heparan sulfate proteoglycans (HSPG) on the surface, thus achieving binding with tumor cells without affecting normal tissues.
Aura’s main candidate product AU-011 is a VDC based on HPV-derived virus-like particles conjugated with photo-activated cytotoxic payloads. Previous preclinical data confirmed that AU-011 can target various tumor types, including choroidal melanoma and non-muscle invasive bladder cancer, showing potential for broad applicability. Currently, this VDC candidate drug has entered Phase II clinical studies for the treatment of primary cancers.

In recent years, the development of conjugated drugs has been very active. In addition to the well-established ADCs and the aforementioned emerging VDCs, AOCs, and PDCs, increasingly novel “combinatorial” forms continue to emerge, including radionuclide conjugates (RDC), small molecule conjugates (SMDC), antibody immune-stimulatory conjugates (ISAC), antibody fragment conjugates (FDC), antibody-cell conjugates (ACC), antibody-oligonucleotide conjugates (AOC), and antibody-biopolymer conjugates (ABC).
In this trend, for pharmaceutical companies, it represents both opportunities and challenges. On one hand, some novel conjugation modalities with the tissue specificity benefits of antibody targeting are evident, providing great support for other therapeutically valuable drugs that are limited by certain barriers. Additionally, these conjugated drugs have shown initial efficacy for different indications, offering various treatment options for patients with huge market potential. On the other hand, for pharmaceutical companies to secure a place in the conjugated drug market, it is crucial to innovate in linkers, effective payloads, and conjugation point technologies, carving out a completely different differentiation path. With deeper research, it is believed that this field will also welcome heavyweight players like “DS-8201.”
References:
1.https://mp.weixin.qq.com/s?__biz=MzkxNzM3ODU0Ng==&mid=2247484932&idx=2&sn=afc1a07b6b4e7398039e672ab4eaacdb&chksm=c140c299f6374b8f959e63315ba2cece6d0438d61c99778ed37bbf794a620bbad3f8099a8c88&mpshare=1&scene=1&srcid=0919nnWzku9M5A4K2KskdTLG&sharer_sharetime=1663554499085&sharer_shareid=7b6ccb0dc4e247ce1c8dd4e8d8238ae6&version=4.0.16.6007&platform=win#rd
2.https://mp.weixin.qq.com/s?src=11×tamp=1663582256&ver=4054&signature=VUqH6VP*o-c1xah*6fCC2j2g7ukdNd7FxQcK1ugiYu1o0Y3VgMlzv2UjKDRJZIAH9gxmpOcX6wGZAMjwJV6RFrrYPDDaFf4GIQoSIKH-54cqh3aHok2PRXH7jObZUeW&new=1
——Recent Popular Events Overview——
▼On September 21, Merck China’s Biosafety Testing Laboratory officially opened.

▼ On September 22, Analysis of Efficient Synthesis Strategies and Process Flows for Oligonucleotide Drugs