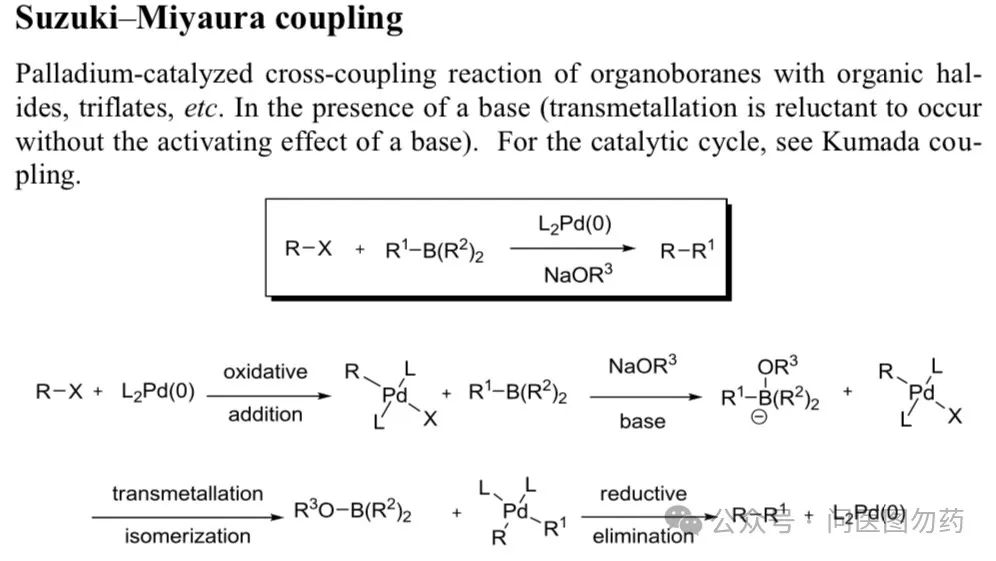
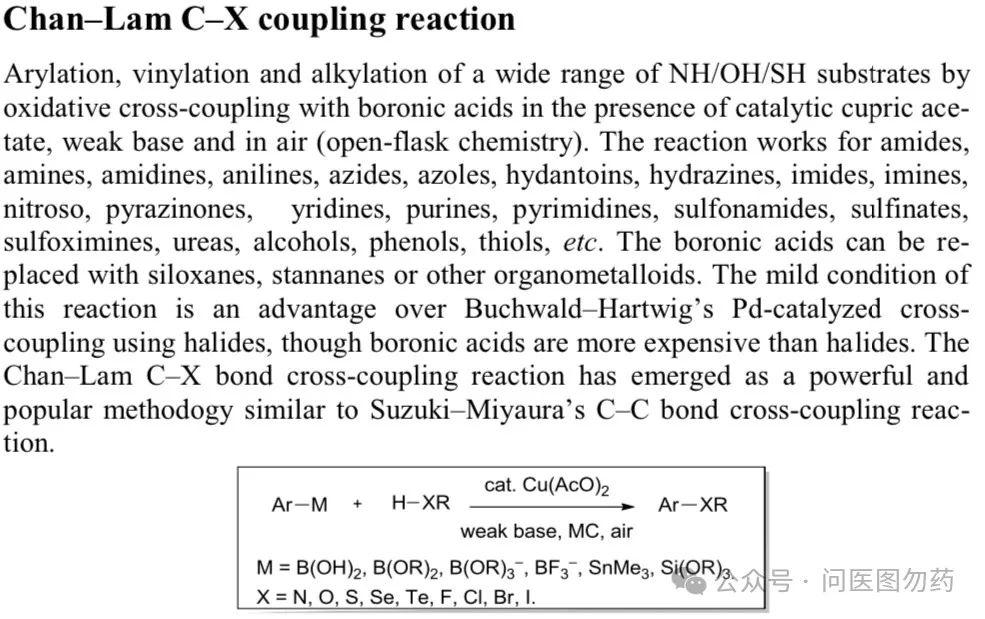
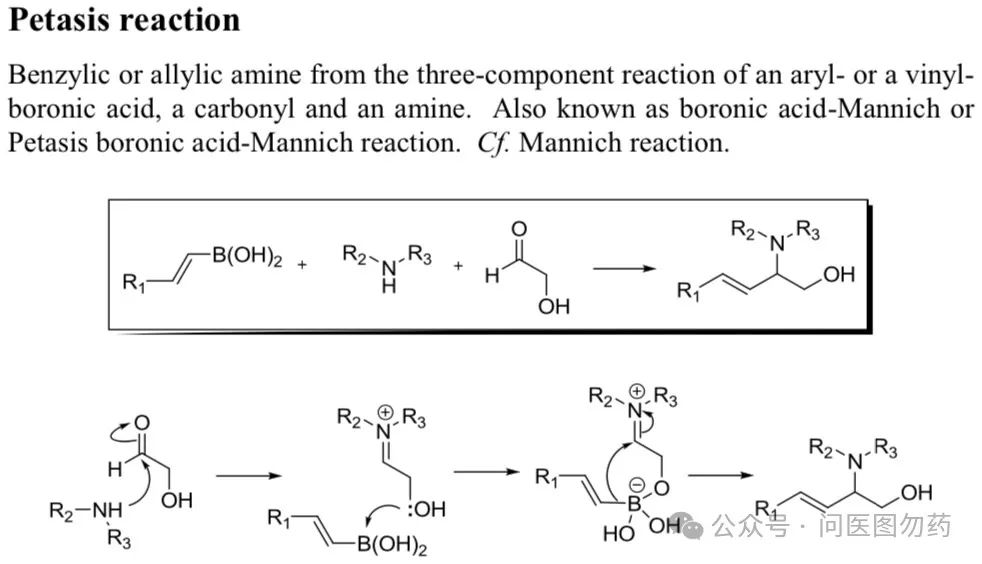
In the previous article, we focused on two monodentate phosphine ligands, (monodentate phosphine BI-DIME and AntPhos) and their applications in Suzuki-Miyaura cross-coupling, detailing their use in C(sp2)-C(sp3) bond coupling. Boronic acids (esters) are important building blocks in organic synthesis, commonly used in Suzuki-Miyaura type C-C coupling, Chan-Lam type C-X (X = N, O, S, etc.) coupling, Petasis boronic acid-Mannich reaction, and the Minisci reaction involving boronic acids and heteroaromatic compounds under oxygen conditions.
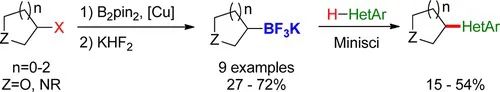
J. Org. Chem. 2013, 78, 9, 4615–4619
With the in-depth research on Suzuki-Miyaura cross-coupling, methods for synthesizing aryl boronic esters have made significant progress. Common methods include: Miyaura borylation reaction, lithiation-borate reaction (Li-X exchange, n-butyllithium deprotonation, etc.), and formate reagent Li-X exchange borylation.
Akira Suzuki and others were the first to attempt to expand the substrate applicability of Suzuki-Miyaura coupling to alkyl boron compounds, reporting the C-C coupling reaction of B-alkyl-substituted 9-borabicyclo[3.3.1]nonane (B-alkyl-9-BBN) with halogenated aromatics/olefins. Since then, alkyl boronic acids (esters) have been given a new mission and are widely used in coupling reactions. This article summarizes several common synthesis methods for bulky vinyl boronic esters. (Next time, we will continue to bring you a summary of synthesis methods for bulky alkyl boronic esters.)
Example 1: Synthesis of bulky vinyl boronic esters via Pd-catalyzed borylation of vinyl halides and their derivatives through the Miyaura borylation reaction.
Considering that vinyl halides have chemical properties similar to aryl halides, we will prioritize the Miyaura borylation reaction to synthesize vinyl boronic esters and their derivatives. Below is a synthesis case of bulky vinyl boronic esters reported by Stephen L. Buchwald et al.
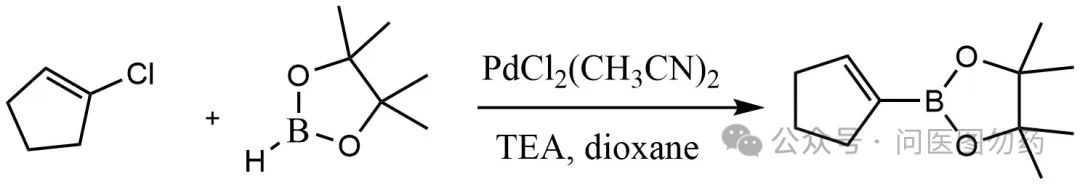
General Procedure for the Pd-Catalyzed Borylation of Aryl Chlorides with Pinacol Borane
An oven-dried resealable Schlenk tube possessing a Teflon screw valve was charged with PdCl2(CH3CN)2 (3.0-4.0%) and SPhos (12.0-16.0%). The Schlenk tube was capped with a rubber septum and then evacuated and backfilled with argon (this sequence was carried out a total of two times). NEt3 (0.500 mL) was added via syringe, through the septum, followed by the addition of the aryl chloride (0.50 mmol) and pinacol borane (0.109 mL, 96.1 mg, 0.75 mmol) in a like manner (aryl halides that were solids were added with the other solid reagents). The septum was then replaced with a Teflon screw valve, and the Schlenk tube was sealed. The reaction mixture was heated to 110°C until the aryl halide had been completely consumed as determined by gas chromatography and was then allowed to cool to room temperature. The reaction solution was filtered through a thin pad of Celite (eluting with ethyl acetate), and the eluent was concentrated under reduced pressure. The crude material so obtained was purified via flash chromatography on silica gel.
References
An Improved System for the Palladium-Catalyzed Borylation of Aryl Halides with Pinacol Borane. J. Org. Chem. 2008, 73, 14, 5589–5591. https://doi.org/10.1021/jo800727s
Example 2: Synthesis of bulky vinyl boronic esters from vinyl halides via lithiation-borylation reaction.
The lithiation-borylation reaction is an efficient pathway for synthesizing bulky vinyl boronic esters, effectively avoiding the use of precious metals like Pd, Rh, etc., while preventing intermolecular cross-coupling. This method has the important advantages of simplicity and a wide substrate applicability.
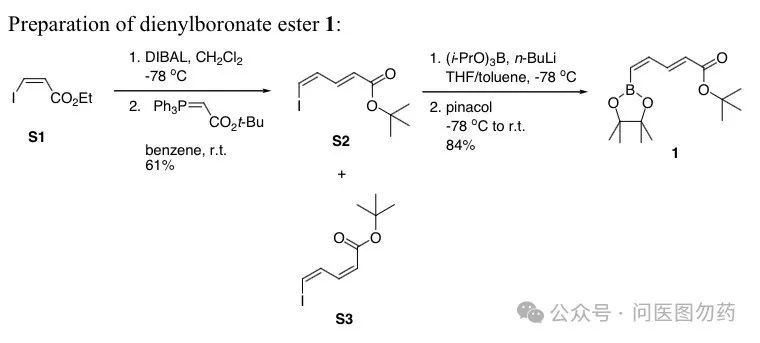
References
Rhodium-Catalyzed Vinylcyclopropanation/Cyclopentenation of Strained Alkenes via a Sequential Carborhodation Process. J. Org. Chem. 2009, 74, 6, 2521–2526. https://doi.org/10.1021/jo900039g
Example 3: Synthesis of bulky vinyl boronic esters via CrCl3 catalyzed reaction of vinyl esters.
In recent years, significant progress has been made in transition metal-catalyzed reductive cross-coupling reactions. Among other transition metals, chromium, as the most abundant sixth group transition metal on Earth, has attracted considerable attention in academia. Xiaoming Zeng et al. from Sichuan University reported a strategy for synthesizing vinyl boronic esters from vinyl trifluoromethanesulfonates (tert-butyl esters) using CrCl3 as a catalyst.
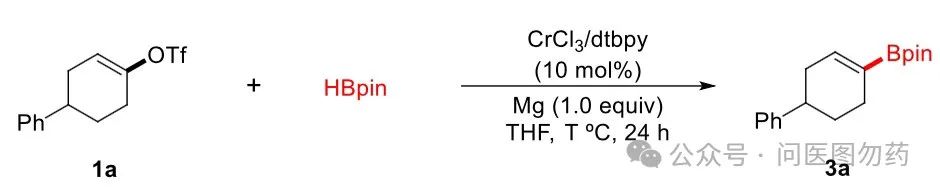
General Procedure for Cr-Catalyzed Selective Borylation of Vinyl Triflates with PinacolboraneIn a dried Schlenk tube were placed vinyl triflates (0.2 mmol), CrCl3 (3 mg, 0.02 mmol), dtbpy (5 mg, 0.02 mmol), Mg (5 mg, 0.2 mmol) and HBpin (58 μL, 0.4 mmol) under atmosphere of nitrogen. After the addition of a freshly distilled THF (1 mL) by syringe, the resulting mixture was stirred at 40°C in an oil bath for 18 h. The volatiles were removed under vacuum, and the crude product was purified by silica gel chromatography to give the corresponding vinyl boronate esters.
References
Chromium-Catalyzed Selective Borylation of Vinyl Triflates and Unactivated Aryl Carboxylic Esters with Pinacolborane. Org. Lett. 2022, 24, 17, 3227–3231. https://doi.org/10.1021/acs.orglett.2c01015
Example 4: Synthesis of bulky vinyl boronic esters involving Ts-hydrazone.
Dennis G. Hall et al. reported a method for synthesizing bulky vinyl boronic esters (cyclic ketone substrates) through the reaction of Ts-hydrazone under the influence of n-butyllithium, releasing one molecule of N2 after the departure of the Ts group, forming a vinyl carbanion that attacks boronic acid pinacol isopropyl ester.
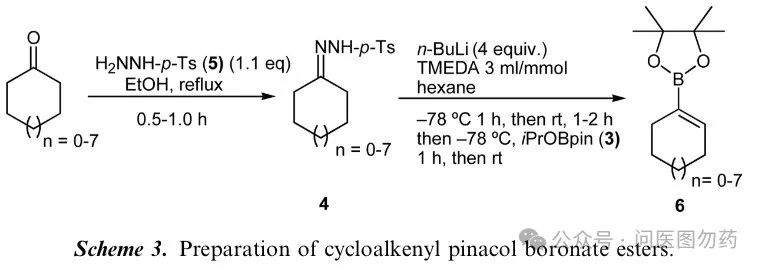
Compound 6a (n=5)
To a flame-dried 500-mL round-bottom flask equipped with a magnetic stirbar and rubber septum, cyclopentanone p-tolylsulfonylhydrazone (6.48 g, 27.15 mmol) was added followed by 80 mL of anhydrous hexanes. To this mixture 80mL of anhydrous TMEDA (3mL/mmol of hydrazone) were added, and the reaction mixture was cooled to -78oC and maintained at this temperature for 15min, after which 43mL (107.5mmol, 4.0 equiv.) of 2.5M n-BuLi was added over 15min. The reaction mixture was then stirred for 1h at -78oC and then brought to room temperature and stirred for 1.5h. Nitrogen was extruded from the reaction, and at the end of the period, the reaction mixture was then brought to -78oC and maintained for another 15min, after which 20.1g (108.6mmol, 4 equiv.) of pinacol isopropyl borate were added. The reaction mixture was stirred for another hour at -78oC and then brought to room temperature and stirred for 3h. The reaction was quenched with the addition of saturated NH4Cl and then extracted three times with ether. The combined organic extracts were dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was subjected to flash chromatography (2% EtOAc/hexanes) to afford 4.19g (80%) of 6a as a light yellow oil. IR (neat) 2979, 1615, 1374, 1318, 1146cm-1; 1H NMR (400MHz, CDCl3) d 6.54 (t, J=2.0Hz, 1H), 2.48–2.31 (m, 4H), 1.88–1.74 (m, 2H), 1.27 (s, 12H); 13C NMR (100MHz, CDCl3) d 147.5, 83.0, 34.8, 34.5, 24.8, 23.9.
References
Convenient Preparation of Cycloalkenyl Boronic Acid Pinacol Esters. Synthetic Communications, 2008, 38, 3984–3995. https://doi.org/10.1080/00397910802245762
Example 5: Synthesis of bulky vinyl boronic esters through palladium-catalyzed Ts-hydrazone via carbene migratory insertion.
In 2021, Jianbo Wang et al. reported a strategy for synthesizing bulky vinyl boronic esters through palladium-catalyzed Ts-hydrazone via carbene migratory insertion. The reaction conditions are mild, with a wide substrate applicability and strong functional group tolerance, suitable for synthesizing di-, tri-, and tetra-substituted vinyl boronic esters and their derivatives.
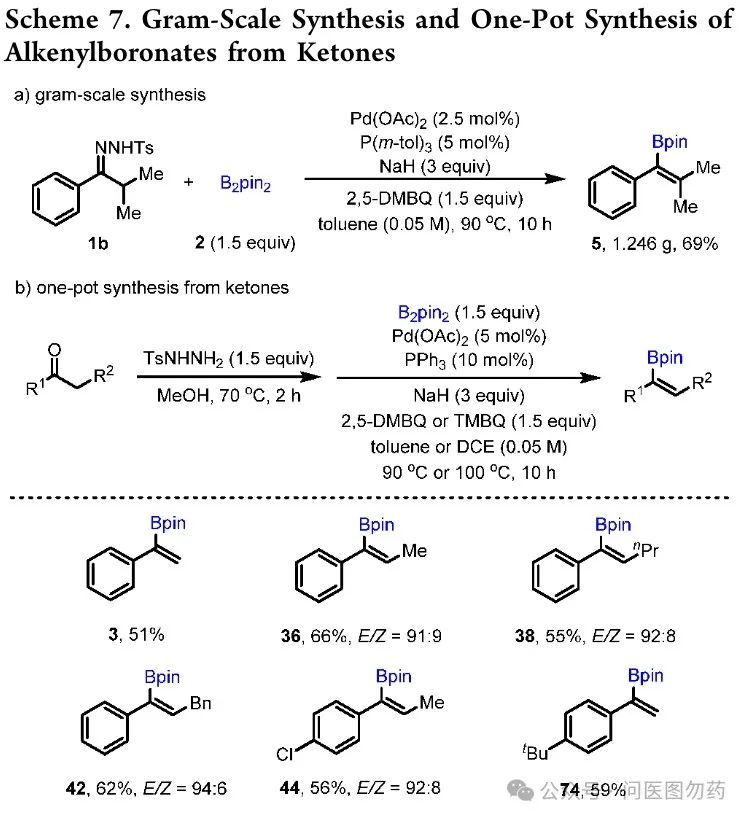
General Procedure for One-Pot Synthesis of Alkenylboronates from Ketones
An oven-dried 25 mL Schlenk flask containing a magnetic stir bar was charged with ketone (0.3 mmol), TsNHNH2 (83.8 mg, 0.45 mmol, 1.5 equiv) and MeOH (1 mL). The reaction was stirred at 70oC for 2 h. After the ketone was completely consumed by TLC monitoring, the solvent was removed in vacuo. Then, Pd(OAc)2 (3.4 mg, 0.015 mmol, 5 mol%), PPh3 (7.9 mg, 0.03 mmol, 10 mol%), 60% NaH (36 mg, 0.9 mmol, 3 equiv), 2,5-DMBQ (61.2 mg, 0.45 mmol, 1.5 equiv), and B2pin2 (114.3 mg, 0.45 mmol, 1.5 equiv) was added to this flask successively. After degassed and filled with N2, toluene (6 mL) was added to the flask via syringe. The mixture was stirred at 90oC for 10 h and then cooled to room temperature. The mixture was filtered through a short plug of silica gel and washed with Et2O as the eluent. The filtrate was concentrated under reduced pressure and purified by silica gel column chromatography to afford the desired product.
References
Synthesis of Alkenylboronates from N-Tosylhydrazones through Palladium-Catalyzed Carbene Migratory Insertion. J. Am. Chem. Soc. 2021, 143, 26, 9769–9780. https://doi.org/10.1021/jacs.1c02331