
First Affiliated Hospital of University of Science and Technology of China
Professor Yan Ji

Device therapy is an important treatment method for heart failure, especially severe heart failure. Currently, the acceptance and prevalence of this therapy in China still lag behind that of developed countries in Europe and America. This article reviews some representative heart failure device therapy technologies and advancements.
Cardiac Resynchronization Therapy
Cardiac Resynchronization Therapy (CRT) is a landmark technology in heart failure device therapy. After nearly 30 years of clinical research and practice, it has been fully proven to effectively improve patients’ heart failure symptoms, exercise tolerance, and quality of life, reverse myocardial remodeling, and reduce heart failure hospitalization and mortality rates. The “2022 American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Failure Society of America (HFSA) Heart Failure Management Guidelines” updated the CRT treatment process for patients with heart failure with reduced ejection fraction (HFrEF) (Figure 1) and conducted a high-quality cost-effectiveness analysis of treatment measures, providing a value statement for treatment recommendations.
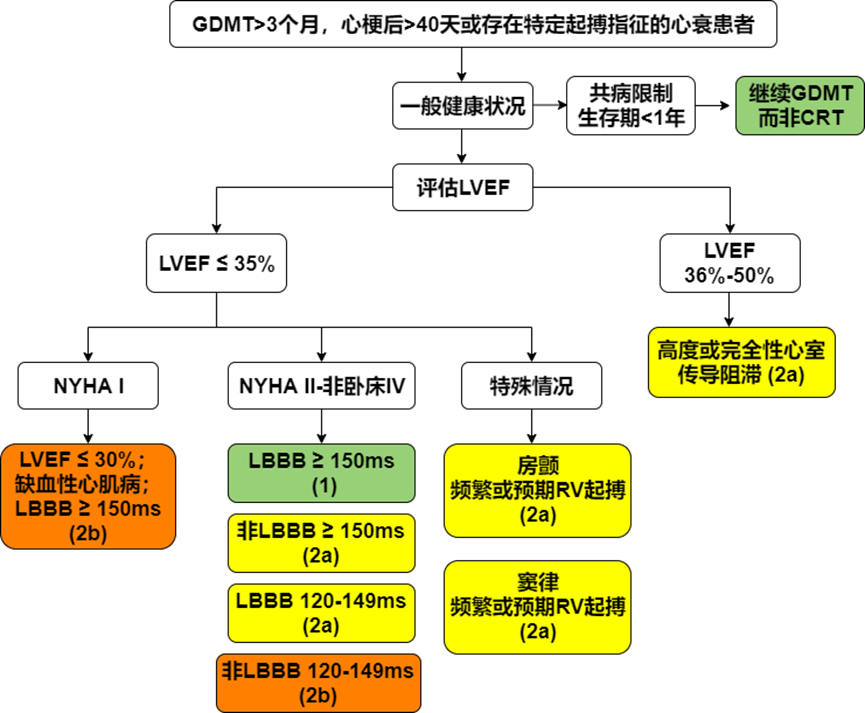
Figure 1 CRT treatment process for HFrEF patients
Compared to traditional biventricular pacing, left ventricular quadripolar leads and multipoint pacing (MPP) can effectively address challenges such as high thresholds, scar areas, and phrenic nerve stimulation, helping to improve CRT response rates. Recently published multicenter, medium- to long-term follow-up studies in China have shown that CRT using left ventricular quadripolar leads and MPP can safely and effectively enhance patients’ echocardiographic and clinical response indicators.
His Bundle Pacing
Approximately 30% to 40% of heart failure patients do not respond to traditional CRT in clinical practice. Directly pacing the His bundle to conduct cardiac electrical activity through the His-Purkinje system can simulate physiological biventricular activation. New resynchronization techniques, including His bundle pacing (HBP) and left bundle branch pacing (LBBP), are becoming effective supplements to traditional CRT. The “2021 European Society of Cardiology (ESC) Guidelines for Cardiac Pacing and Cardiac Resynchronization Therapy” recommends the standardized use of HBP: 1. In patients receiving HBP treatment, it is recommended to program the device according to the specific requirements of HBP (Class I recommendation); 2. For CRT patients with failed coronary sinus electrode implantation, choosing HBP or other techniques (such as surgical left ventricular epicardial pacing) is reasonable (Class IIa recommendation); 3. In patients receiving HBP treatment, it may be considered to implant a right ventricular electrode as a backup pacing electrode under specific circumstances (Class IIa recommendation). The “Chinese Expert Consensus on His-Purkinje System Pacing” further points out that despite the lack of sufficient clinical evidence, it is reasonable to perform rescue His-Purkinje system pacing in cases of failed left ventricular lead implantation and non-response to CRT, both theoretically and clinically.
Compared to HBP, LBBP has similar clinical benefits, is relatively simple to perform, can pace beyond the block site, has a low pacing threshold, and a relatively stable long-term threshold. It can capture local myocardium at lower outputs, making it safer and currently more promising for application in China. Recently, domestic scholars completed the world’s first head-to-head comparison study of LBBP-CRT and traditional CRT, the LBBP-RESYNC study, which found that in non-ischemic heart failure patients with LBBB, LBBP-CRT significantly improved left ventricular ejection fraction (LVEF) at 6 months post-operation compared to traditional CRT, promoting left ventricular reverse remodeling. This finding provides a research basis for future larger-scale randomized controlled trials (RCTs) with hard endpoints.
Implantable Cardioverter-Defibrillators and New Technologies
Implantable cardioverter-defibrillators (ICDs) are important means of primary and secondary prevention of sudden cardiac death (SCD) in heart failure patients. Guidelines in Europe and America recommend ICD primary prevention for patients meeting the following criteria (Class I recommendation): ischemic heart failure, New York Heart Association (NYHA) functional classification II-III symptoms, LVEF ≤ 35%, optimal medical therapy for ≥ 3 months (2021 ESC) or “chronic” (2022 ACC/AHA/HFSA), more than 40 days post-myocardial infarction (MI), and expected survival of > 1 year. However, there are differences in the recommendation levels for primary prevention in non-ischemic heart failure patients between the 2022 AHA/ACC/HFSA guidelines (Class I recommendation) and the 2021 ESC guidelines (Class IIa recommendation).
In recent years, the development of new technologies such as subcutaneous implantable ICDs (S-ICD) and extravascular implantable ICDs (EV-ICD) has received widespread attention. Recent studies have shown that S-ICD has a similar defibrillation efficacy to traditional transvenous ICDs (TV-ICD) and trends towards reducing lead-related complications. Preliminary results from the 5-year follow-up of the S-ICD PAS study published by AHA 2022 further demonstrate the long-term safety and efficacy of S-ICD. The electrode lead of EV-ICD is implanted behind the sternum, closer to the heart than S-ICD, providing bradycardia pacing and ATP functions, and has advantages such as small size, low defibrillation threshold, and long lifespan (10-12 years). The recently published EV-ICD Pivotal trial demonstrated the safety and efficacy of EV-ICD, finding that the defibrillation success rate reached 98.7%, with no major complications reported during the perioperative period, and Kaplan-Meier estimates showed that 92.6% of patients had no major device or procedure-related complications within 6 months.
Cardiac Contractility Modulation
Cardiac contractility modulation (CCM) enhances myocardial contractility by delivering electrical pulses through two right ventricular electrode leads during the absolute refractory period of the ventricular myocardium, increasing calcium ion influx into myocardial cells. CCM was approved for clinical use by the U.S. Food and Drug Administration (FDA) in 2019 and received domestic market approval from the National Medical Products Administration (NMPA) in 2021.
Unlike traditional CRT, CCM can be used to treat heart failure patients with narrow QRS complexes. The applicable population includes NYHA II-IV patients with HFrEF and QRS duration < 130 ms. Studies such as FIX-HF-3, FIX-CHF-4, and FIX-HF-5 have shown that CCM can improve exercise tolerance and quality of life in heart failure patients. Subsequent results from the FIX-HF-5C study indicated that CCM benefits specific heart failure populations with NYHA III-IV and LVEF 25%-45%, observing a significant reduction in the composite endpoint of cardiovascular death and heart failure hospitalization at 6 months post-operation, primarily due to a decrease in heart failure hospitalization events. However, the long-term efficacy of CCM currently lacks support from large-scale RCT evidence. Additionally, a prospective, multicenter, randomized, quadruple-blind AIM HIGHer trial is underway to evaluate the safety and efficacy of CCM in heart failure patients with preserved ejection fraction (HFpEF).
Edge-to-Edge Mitral Valve Repair
Transcatheter edge-to-edge mitral valve repair (TEER) has unique advantages such as minimal trauma and rapid recovery, providing a new option for treating heart failure-related secondary mitral regurgitation (MR). Based on the results of the COAPT trial, the 2022 AHA/ACC/HFSA guidelines detail the application value of TEER in heart failure patients with MR. Specifically, TEER has clinical benefits in patients who have undergone guideline-directed medical therapy (GDMT) but continue to experience symptoms, with transesophageal echocardiography (TEE) showing suitable mitral valve anatomy, LVEF 20%-50%, LVESD ≤ 70 mm, and PASP ≤ 70 mmHg (Class IIa recommendation).
Other Heart Failure Device Therapy Technologies
Techniques such as autonomic nervous modulation therapy, vagus nerve stimulation (VNS), spinal cord stimulation (SCS), and baroreceptor activation therapy (BAT) have theoretical and small-sample research feasibility for treating chronic heart failure, but lack large-scale clinical trials to demonstrate their hard endpoint efficacy.
Autonomic Nervous Modulation Therapy
The autonomic nervous system (ANS) plays a crucial role in the regulation and homeostasis of the cardiovascular system. The ANS is mainly composed of the sympathetic and parasympathetic nervous systems, both of which jointly govern the heart. In heart failure, the neurohumoral regulatory mechanism is activated, characterized by sympathetic excitation and reduced vagal activity. Persistent ANS imbalance promotes ventricular remodeling and the progression of heart failure, while the progression of heart failure further exacerbates the differences in sympathetic and vagal activity.
Vagus Nerve Stimulation
Schwartz et al. first reported the feasibility of long-term vagus nerve stimulation (VNS) for treating chronic heart failure. The study found significant improvements in NYHA functional classification, quality of life scores, and left ventricular end-diastolic volume (LVEDV) in patients post-VNS. However, clinical trials to date have failed to reliably reproduce the advantages of VNS in treating heart failure. The NECTAR-HF trial observed improvements in NYHA functional classification and quality of life scores post-VNS, but no significant improvements in LVEDV (the primary endpoint) and other echocardiographic parameters at 6 months post-operation. The ANTHEM-HF trial assessed the feasibility of left or right VNS for HFrEF, finding that 77% of patients improved in NYHA functional classification at 6 months post-operation, with improvements in LVEF, LVEDV, and LVESD. Larger-scale INOVATE-HF trials also observed improvements in NYHA functional classification, quality of life scores, and 6-minute walk distance post-operation, but no significant differences in LVEDV compared to the control group. Studies indicate that VNS does not reduce all-cause mortality or heart failure events in chronic heart failure patients. The ongoing ANTHEM-HFrEF trial will further clarify the long-term efficacy of VNS in treating heart failure. Additionally, non-invasive VNS offers new ideas for heart failure treatment; a small-sample prospective, randomized, double-blind, 2×2 crossover study showed that 1 hour of auricular vagus nerve stimulation improved overall left ventricular longitudinal strain and heart rate variability in HFrEF patients.
Spinal Cord Stimulation Therapy
Spinal cord stimulation (SCS) devices consist of an implanted pulse generator connected to one or more epidural leads, delivering electrical pulses to the dorsal spinal cord, previously used for chronic pain treatment. Early studies suggested that SCS might help regulate myocardial blood flow and reduce catecholamine production. Currently, SCS treatment for heart failure lacks support from large-scale clinical trials. A prospective, randomized, double-blind, crossover pilot trial by Torre-Amione G et al. found improvements in heart failure symptoms post-SCS, but no improvements in cardiac structure or heart failure biomarkers. The SCS HEART study observed improvements in NYHA functional classification, quality of life scores, and LVEDV post-SCS. However, the DEFEAT-HF study found no clinical benefits of SCS treatment for heart failure.
Baroreceptor Activation Therapy
Baroreceptor activation therapy (BAT) devices consist of an implanted pulse generator and carotid sinus electrodes, stimulating the carotid sinus with electrical pulses to simultaneously increase vagal activity and decrease sympathetic activity. Recent BeAT-HF trials demonstrated that BAT is safe in HFrEF patients, improving exercise tolerance, quality of life scores, and reducing NT-proBNP levels.
Conclusion
In recent years, significant progress has been made in device therapy for heart failure, providing more options and evidence support for symptom improvement, quality of life enhancement, and survival benefits for heart failure patients. However, the promotion and popularization of new technologies and concepts in device therapy still face significant challenges in China. Encouragingly, an increasing number of domestic scholars are proposing Chinese solutions and providing Chinese evidence in this field.
 ENDPrevious Review # How should cardiovascular disease patients optimally select treatment medications during the COVID-19 pandemic?# How to control ventricular rate in atrial fibrillation?#
ENDPrevious Review # How should cardiovascular disease patients optimally select treatment medications during the COVID-19 pandemic?# How to control ventricular rate in atrial fibrillation?#
AHA releases the top ten research advances in cardiovascular field for 2022
More Selected Content
