Assessing the positive or negative valence of external sensory cues and translating them into appropriate behavioral responses is crucial for animal survival in complex or challenging environments. Negative valence signals lead to aversive/avoidance behaviors, while positive valence signals promote reward/approach behaviors. Much previous research has focused on the neural structures within the emotional processing network and how different cell populations influence aversive or reward behaviors, but there has been little research on how sensory signals are transmitted to these structures.
Currently, the lateral habenula (LHb) has been identified as a key brain region regulating reward/aversive responses in studies of the emotional processing network. The LHb mainly contains excitatory glutamatergic neurons that can modulate the dopamine (DA) and serotonin (5-HT) systems. Studies have found that these neurons can be activated by aversive signals and inhibited by reward signals, resulting in opposite responses. Several input structures upstream of the LHb, such as the ventral pallidum and lateral preoptic area, project excitatory and inhibitory axons to the LHb.Excitatory projections can activate the LHb, which then partially exerts negative regulation on the DA and 5-HT systems via GABAergic projections from the interpeduncular nucleus, leading to aversive or anti-reward effects. Conversely, inhibitory projections have positive valence, reducing LHb activity and resulting in reward-related emotional effects. Thus, by receiving converging positive and negative valence signals, the LHb can serve as a hub that integrates information from various sources.
The medial septum (MS) has been found to transmit bottom-up aversive sensory signals to the LHb through its glutamatergic neurons, leading to negative emotion-related behaviors[1]. Although GABAergic neurons in the MS have also been found to influence LHb neurons, their functional roles in different behavioral contexts remain unclear. The MS contains different types of GABAergic neurons, including those expressing parvalbumin (PV) and somatostatin (SOM), which may receive different inputs and form intra-nuclear connections with glutamatergic neurons. One possibility is that certain specific types of MS GABAergic neurons may receive and convert reward information from different sensory pathways to regulate appetitive behaviors.

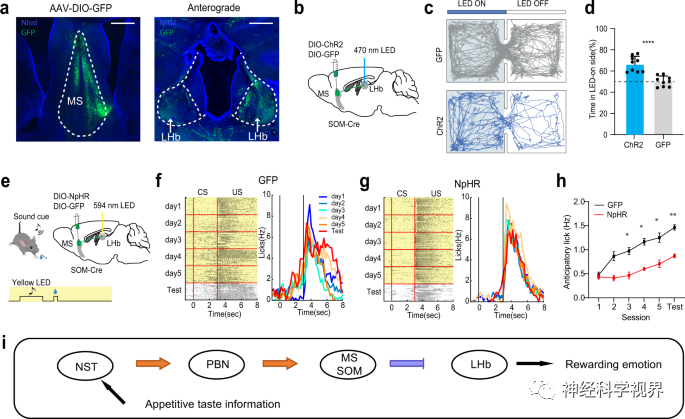
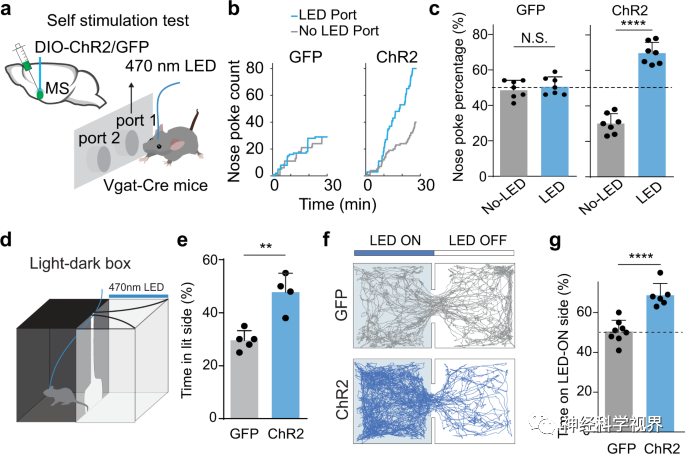
The researchers used Vgat-Cre mice and activated GABAergic neurons in the MS by injecting Cre-dependent channelrhodopsin-2 (ChR2) adeno-associated virus (AAV). They first employed a bidirectional self-stimulation paradigm to assess the reinforcing value associated with activation. Whenever the animal’s nose poked at the designated LED on the port, LED stimulation would be triggered, while poking at another port would not trigger any light stimulation (Figure 1a). During a 30-minute testing period, experimental mice showed a significantly higher number of pokes at the LED port compared to the control port; however, control mice expressing only GFP showed no significant difference in pokes between the two ports (Figure 1b, c). This result indicates that acute optogenetic activation of MS GABAergic neurons enhances self-stimulation behavior, which is an important hallmark of reward-encoding neurons. Furthermore, in the light-dark box test, animals expressing ChR2 spent significantly more time in the light chamber paired with LED stimulation than GFP control mice (Figure 1d, e). In the real-time place preference (RTPP) test, researchers found that Vgat-Cre animals expressing ChR2 spent more time in the stimulation chamber (Figure 1f, g). These results indicate that MS GABAergic neurons encode positive motivational value.
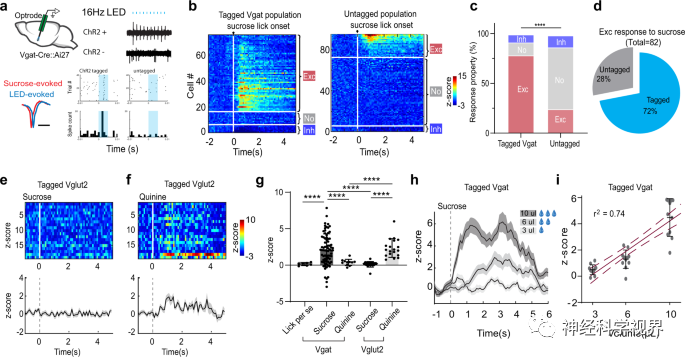
Next, the researchers used optoelectronic recording on head-fixed awake mice licking sugar water to directly detect whether MS GABAergic neurons could respond to reward signals (Figure 2a). For ChR2-labeled (GABAergic) neurons, an increased firing rate was observed in the majority of recorded neurons (79%) after licking, while 13% showed no change in firing rate, and 8% showed decreased firing rate (Figure 2b, c). Among all the recorded neurons that exhibited excitatory responses to sucrose, 72% were labeled with ChR2 (Figure 2d). These results indicate that licking sucrose preferentially activates the GABAergic population. Additionally, similar recordings were performed on mice that were forced to consume quinine (a bitter agent), with no significant changes in firing rate observed (Figure 2g). In contrast to GABAergic neurons, Vglut2-Cre mice labeled MS glutamatergic neurons showed no response to sucrose consumption but were activated by quinine (Figure 2e-g), consistent with previous findings that the activity of these neurons encodes aversive emotional outcomes. In summary, these results suggest that MS GABAergic neurons are preferentially activated by reward signals. More importantly, their response to sucrose was enhanced with increased intake (Figure 2h, i), indicating that activity levels can encode reward value.
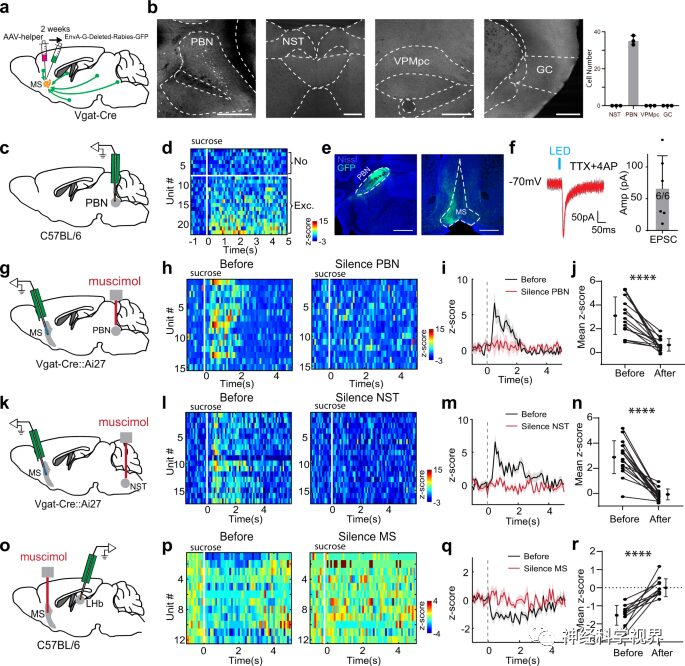
Previously, sucrose-induced inhibitory responses could be observed in LHb neurons. In vivo recordings showed that the inhibitory response was largely reversed by silencing the MS (Figure 3o-r). Thus, the MSGABAergic projections to the LHb mediate the reward-related inhibition in the LHb. These data reveal a bottom-up pathway for transmitting appetitive taste information: from NST to PBN, then to MS GABAergic neurons, which subsequently inhibit LHb neurons.
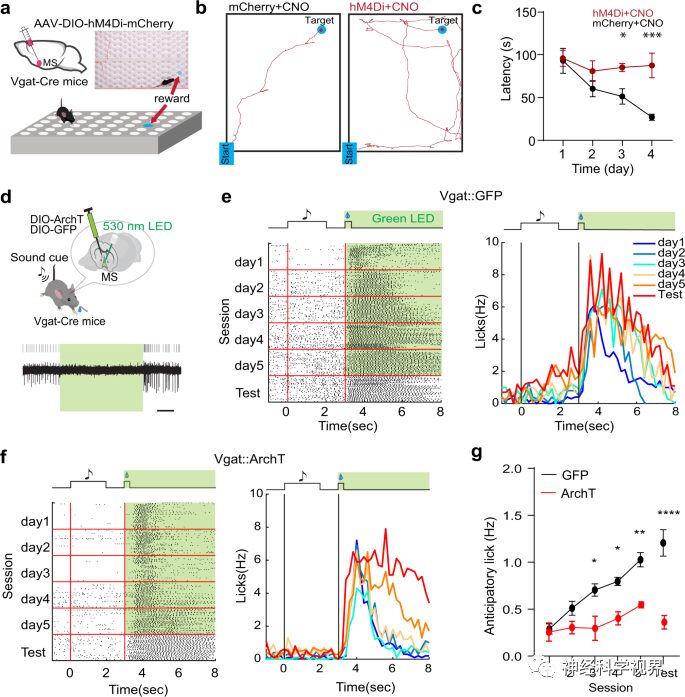
Subsequently, using a cue-reward associative learning paradigm, mice learned to lick sucrose (the reward) after hearing a sound. Since MS GABAergic neurons can encode reward value, they may provide the unconditioned stimulus (US) signal for this associative learning. After silencing MS GABAergic neurons in Vgat-Cre mice using optogenetics (Figure 4d), the researchers conducted training to couple the conditioned stimulus (CS) and US (Figure 4e). Monitoring revealed that licking behavior significantly decreased after inhibiting GABAergic neurons (Figure 4f, g), further indicating that the US response of MS GABAergic neurons is necessary for forming cue-reward associations.
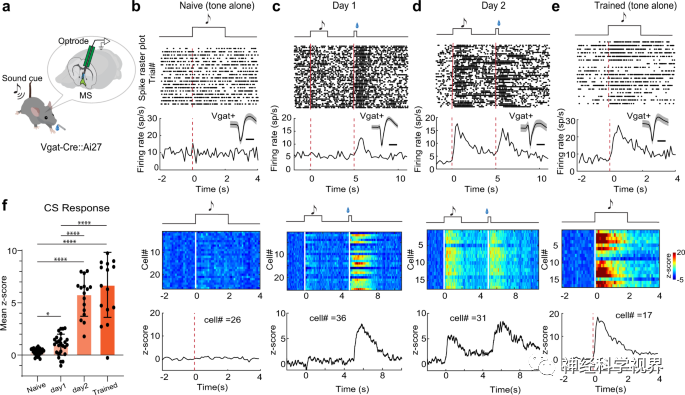
To test how the response of MS GABAergic neurons is formed through learning, the researchers conducted two days of electrode recordings before, during, or after training (Figure 5a). In mice prior to training, no significant response of GABAergic neurons to the CS (tone) was observed (Figure 5b, f). On the first day of training, neurons exhibited a strong response to sucrose (US) while showing a weak response to the CS (Figure 5c, f). On the second day, the response to the CS became strong (Figure 5d, f). After two days of training, a strong response was maintained only to the CS stimulus (Figure 5e, f). On average, the response to the CS increased by 5.8 times compared to the first day (Figure 5f). These results demonstrate the plasticity of MS GABAergic neurons’ responses to reward predictive cues induced by learning.
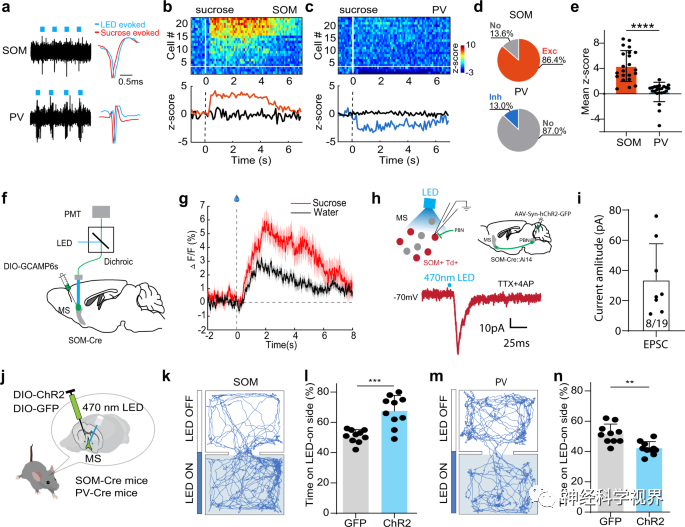
Next, the researchers further distinguished the roles of GABAergic neuron subtypes in reward learning. They expressed Cre-dependent ChR2 in the MS of PV-Cre or SOM-Cre mice and performed optoelectronic recordings (Figure 6a). The experiment found that most SOM neurons were activated by sucrose (Figure 6b, d, e), while most PV neurons showed no response to sucrose (Figure 6c-e). Similarly, the response of SOM neurons to sucrose was stronger than their response to water, as indicated by the Ca2+ signals recorded via fiber photometry (Figure 6f, g), suggesting that they can encode reward value. The researchers further confirmed that SOM neurons receive excitatory monosynaptic input from the PBN (Figure 6h, i). Additionally, in the RTPP test, activation of SOM neurons produced location preference (Figure 6j-l), while activation of PV neurons led to weak location avoidance (Figure 6m, n). These results indicate that SOM neurons encode positive valence and transmit reward information; conversely, PV neurons encode negative valence and are less likely to participate in reward learning.
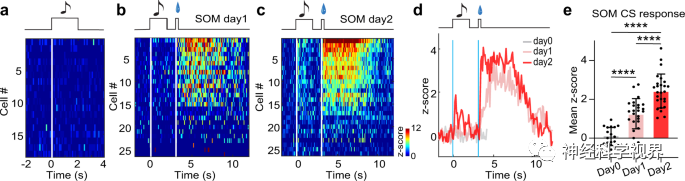
In the Pavlovian conditioning paradigm, the researchers detected the spike activity of SOM neurons before and during reward associative learning. Prior to conditioning, SOM neurons showed no response to the CS tone (Figure 7a), while on the first and second day of conditioning, their response to the CS tone gradually appeared and strengthened (Figure 7b-e), indicating that the SOM subtype of MS GABAergic neurons participates in reward associative learning.
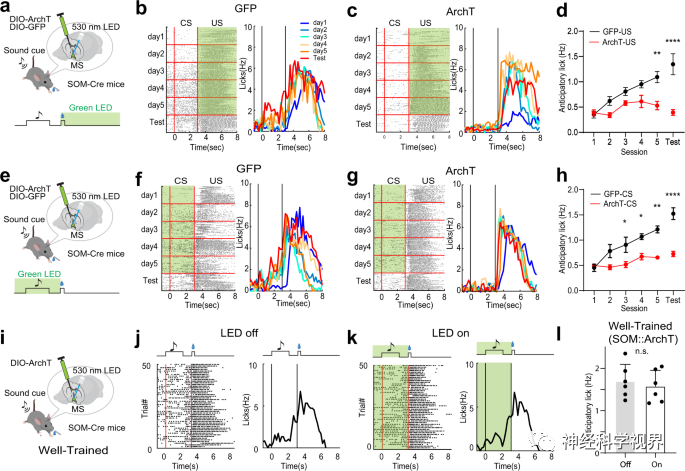
Subsequently, the researchers expressed Cre-dependent ArchT in the MS of SOM-Cre mice to perform optogenetic inhibition during the US window (Figure 8a-d) or the CS+ delay window (Figure 8e-h). Compared to the control group, the inhibition of SOM neurons during CS or US-related activities largely impaired reward associative learning (Figure 8d, h). Interestingly, in mice post-training, optogenetic inhibition of SOM neurons during the CS+ delay window did not affect licking behavior(Figure 8i–l), indicating that after acquiring associative learning, the activity of SOM neurons is not required to express cue-reward associations. In summary, SOM neurons in the MS are essential for acquiring reward associative learning.
By silencing MS to block inhibitory reward responses in the LHb (Figure 3o–r), it suggests that MS SOM neurons may mediate reward associative learning via the LHb. The researchers found a strong projection from MS SOM neurons to the LHb through anterograde tracing (Figure 9a). In the RTPP test, light activation induced location preference, indicating that the projection from MS SOM to LHb is rewarding (Figure 9c, d). Additionally, optogenetic inhibition of the MS SOM→LHb projection led to impaired reward associative learning (Figure 9e-h). These data suggest that MS SOM neurons indeed regulate reward associative learning through projections to the LHb.