First Author: Solveig S. Aamlid, University of British Columbia, Stewart Blusson Quantum Matter Institute.
Corresponding Author: Alannah M. Hallas, from the University of British Columbia, Stewart Blusson Quantum Matter Institute, Department of Physics and Astronomy, University of British Columbia
1. Definition and Characteristics of High-Entropy Materials
The definition of high-entropy materials is not yet unified, but it is generally believed that three conditions must be met: crystallinity, configurational disorder, and phase purity. Crystallinity refers to the material having a crystal lattice with well-defined space group and point group symmetry; configurational disorder is characterized by multiple elements sharing the same lattice site; phase purity requires the material to be a single phase rather than a multiphase mixture. The configurational entropy of high-entropy materials can be calculated using a formula, reaching its maximum when elements are present in equimolar ratios. For example, Figure 1 illustrates the crystal structure characteristics of high-entropy oxides and their random distribution properties, emphasizing the importance of verifying these characteristics through various experimental methods. In high-entropy oxides, this random distribution of metal cations spans the ordered crystal lattice, resulting in significant configurational disorder across all length scales from atomic to macroscopic. To verify whether a material meets the characteristics of high-entropy materials, a series of experimental techniques must be employed, including real-space and reciprocal-space methods, such as X-ray diffraction, electron microscopy, and atom probe tomography, which can sensitively detect the material structure at different length scales.
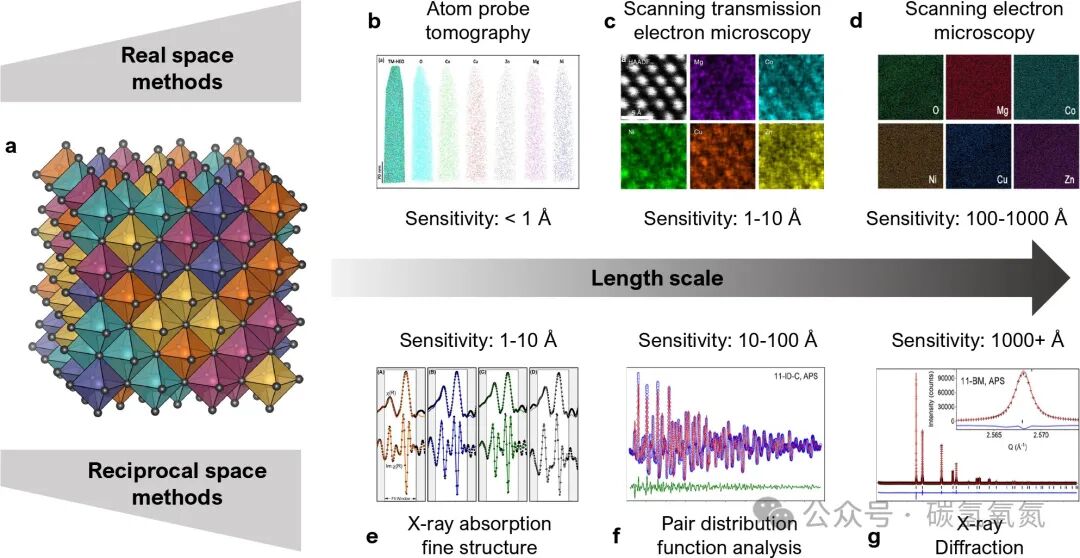 Figure 1: (a) The characteristic of high-entropy oxides (HEOs) is the random distribution of metal cations in an ordered crystal lattice, illustrated here with five different elements in a rock salt structure using VESTA. A series of experiments validating these characteristics through (b − d) real-space and (e − g) reciprocal-space methods, which are sensitive to different length scales. All representative data shown in this figure were collected on the prototype rock salt HEO (Mg,Co,Ni,Cu,Zn)O.
Figure 1: (a) The characteristic of high-entropy oxides (HEOs) is the random distribution of metal cations in an ordered crystal lattice, illustrated here with five different elements in a rock salt structure using VESTA. A series of experiments validating these characteristics through (b − d) real-space and (e − g) reciprocal-space methods, which are sensitive to different length scales. All representative data shown in this figure were collected on the prototype rock salt HEO (Mg,Co,Ni,Cu,Zn)O.
2. The Key Role of Entropy in High-Entropy Oxides
-
Stabilizing New Phases
In high-entropy oxides, entropy plays a crucial role in stabilizing new phases. For example, the rock salt material (Mg,Co,Ni,Cu,Zn)O, which has a single-phase crystal structure, is said to be stabilized by entropy. As the number of elements increases from four to five, the material is more likely to form a single phase, indicating that entropy plays a key role in stabilizing new phases. Furthermore, studies have shown that when the formation enthalpy is positive, the formation of the material must overcome unfavorable enthalpy changes, and the contribution of entropy allows the material to form at high temperatures. Figure 2 shows the variation of ideal configurational entropy with the number of components, as well as the cycling stability and thermal conductivity characteristics of the five-component rock salt HEO in lithium-ion battery applications, highlighting the role of entropy in stabilizing new phases and enhancing functional properties. Specifically, as the number of components increases, the ideal configurational entropy of HEOs also increases, providing a higher thermodynamic driving force for the stability of the material. For instance, in lithium-ion battery applications, the five-component rock salt HEO exhibits a higher capacity retention rate during cycling, indicating a significant improvement in structural stability during charge and discharge processes.
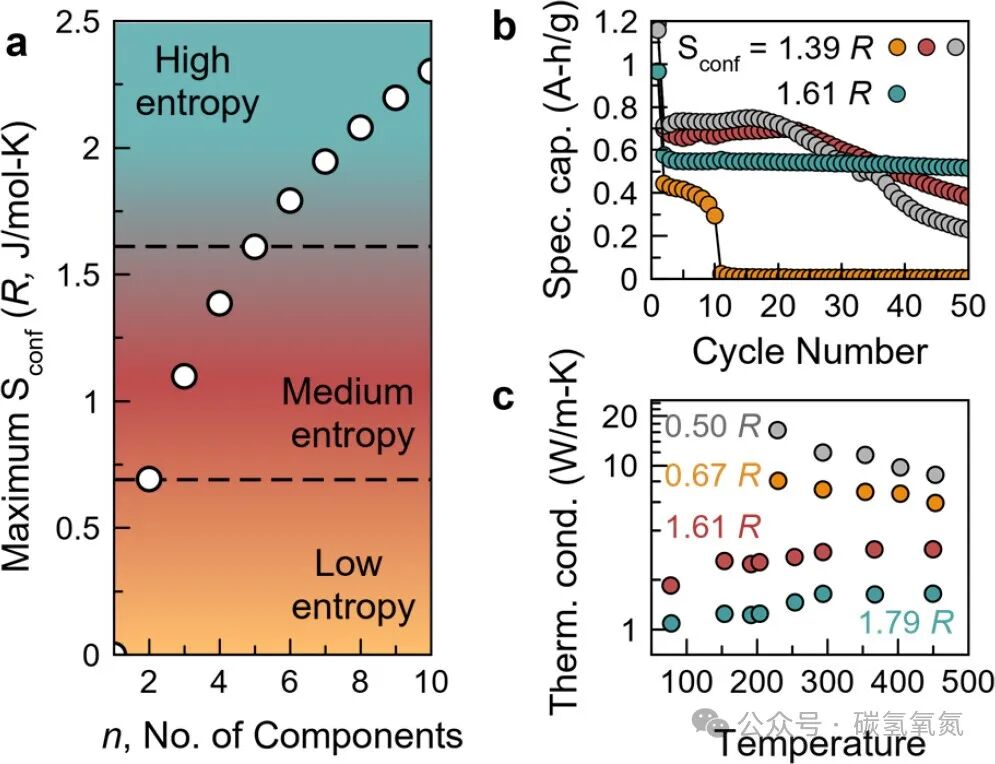
Figure 2: (a) The change of ideal configurational entropy with the number of components n at equimolar ratios, showing the boundary between low and medium entropy at Sconf = 0.69R, and the boundary between medium and high entropy at Sconf = 1.61R. It can be seen that high entropy requires at least five component elements to share a crystallographic position to be realized. The cycling stability of rock salt high-entropy alloys in (b) lithium-ion battery applications, and (c) the suppression of thermal conductivity in thermal barrier coatings.
-
Enhancing Functional Properties
High-entropy oxides often exhibit superior functional properties. In lithium-ion battery applications, the cycling stability of the five-component rock salt HEO is significantly better than that of four-component analogs. This indicates that the configurational disorder of high-entropy oxides contributes to enhancing the performance of the material. Additionally, as the number of components increases, the thermal conductivity of HEOs can be further reduced, providing potential materials for applications such as thermal barrier coatings. Figure 2 illustrates the significant enhancement of functional properties of HEOs with the increase in the number of components. For example, in terms of thermal conductivity, by introducing a sixth component element, the thermal conductivity can be further reduced by twofold, making HEOs potentially valuable in thermal management applications. This reduction in thermal conductivity is primarily attributed to the disordered distribution of cations and local distortions within the material, which together suppress phonon propagation.
3. Symmetry and Symmetry Breaking in High-Entropy Oxides
High-entropy oxides tend to form high-symmetry structures, such as cubic rock salt, fluorite, perovskite, and spinel structures. This symmetry is beneficial for the stability and performance optimization of the material. However, on a local scale, due to element mixing and interactions, symmetry breaking phenomena may occur, such as Jahn-Teller distortions. These local distortions, while not significantly affecting the overall symmetry, can have important impacts on the electronic and optical properties of the material. Figure 3 presents evidence of entropy stabilization, including endothermic reaction enthalpy, reversible phase transitions, the formation of crystal structures, and the theoretical determination of electronic entropy, emphasizing the relationship between symmetry and symmetry breaking in high-entropy oxides. For instance, regarding endothermic reaction enthalpy, calorimetric methods have verified that the formation reaction of high-entropy oxides is an endothermic process, indicating that entropy plays a key role in the formation of the material. In terms of reversible phase transitions, X-ray diffraction has observed the phase transition behavior of the material at different temperatures, further confirming the characteristics of entropy stabilization.
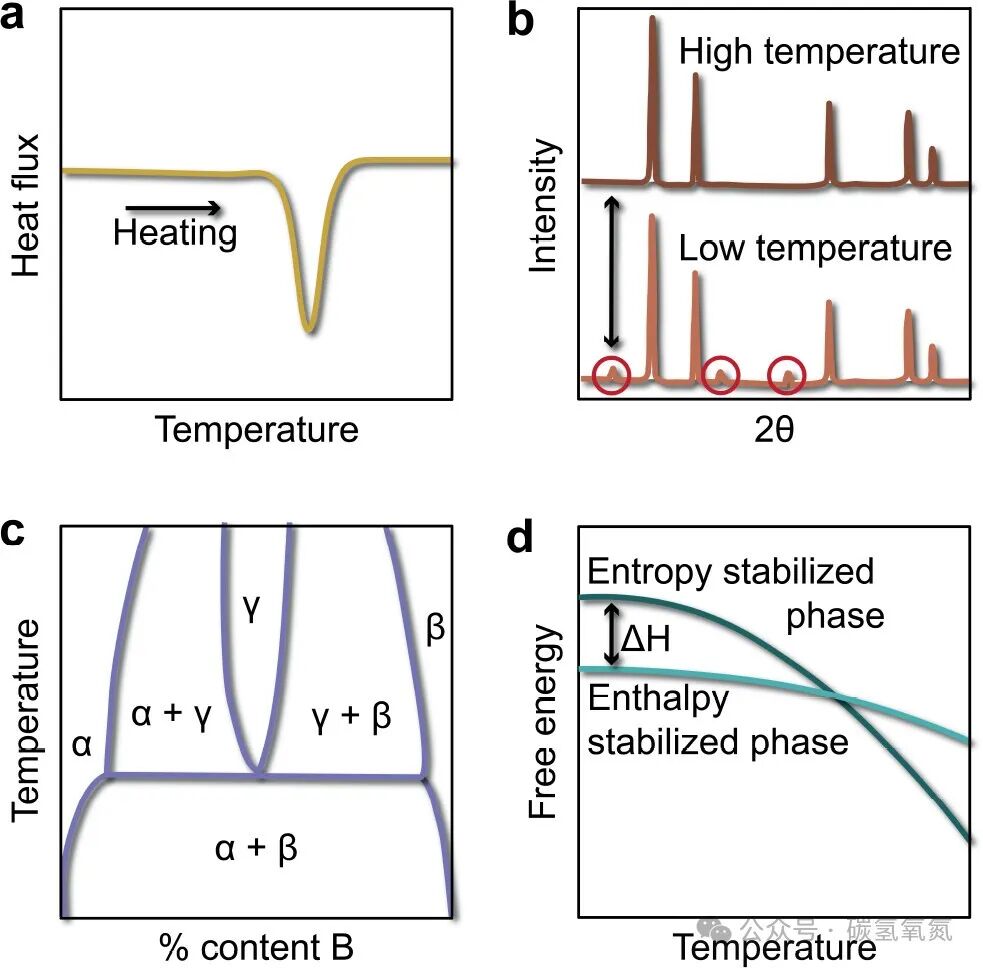 Figure 3: (a) Evidence of endothermic formation enthalpy verified by calorimetric methods; (b) proof of the reversibility of phase transitions through the appearance of impurity peaks in X-ray diffraction after heat treatment below the phase transition temperature; (c) formation of a cation disordered phase that is different from any compositional phase in the structural diagram; (d) using DFT calculations to ensure that the configurational entropy will dominate the free energy at a certain temperature below the melting point by calculating the ΔH of two competing entropy-stabilized and enthalpy-stabilized phases.
Figure 3: (a) Evidence of endothermic formation enthalpy verified by calorimetric methods; (b) proof of the reversibility of phase transitions through the appearance of impurity peaks in X-ray diffraction after heat treatment below the phase transition temperature; (c) formation of a cation disordered phase that is different from any compositional phase in the structural diagram; (d) using DFT calculations to ensure that the configurational entropy will dominate the free energy at a certain temperature below the melting point by calculating the ΔH of two competing entropy-stabilized and enthalpy-stabilized phases.
4. Local Environment and Entropy Enhancement in High-Entropy Oxides
The local environment in high-entropy oxides contributes significantly to the entropy of the material. The distribution and interactions of cations in the oxygen sublattice can affect the configurational entropy of the material. Studies have found that the disordered distribution of cations in the local environment can enhance the entropy of the material, thereby improving its stability and performance. Additionally, point defects such as vacancies can also impact the entropy of the material, subsequently affecting its functional properties. Figure 4 categorizes HEOs based on the magnitude of configurational entropy and the role of entropy in stabilizing their observed crystal structures, illustrating the influence of the local environment on entropy enhancement. For example, in the local environment, smaller cations may lead to the contraction of oxygen coordination polyhedra, while larger cations may cause expansion. These local distortions not only increase the configurational entropy of the material but may also impact the electronic structure and magnetic properties of the material.
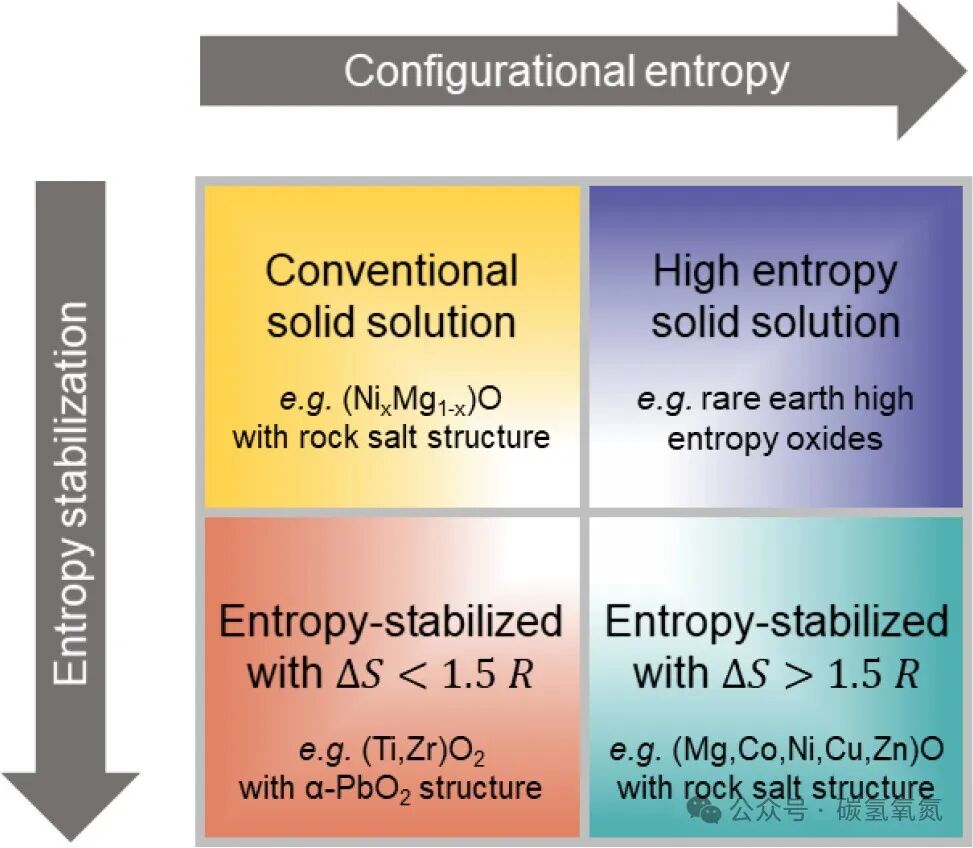
Figure 4: HEOs can be classified based on two related but distinct features: the magnitude of configurational entropy and the role of entropy in stabilizing their observed crystal structures. This figure represents schematically, with configurational entropy increasing from left to right, and the degree of entropy stabilization increasing from top to bottom. Known materials can be classified into each of the four quadrants. Traditional solid solutions have low configurational entropy and are not entropy-stabilized, while HEOs achieve high thresholds either by exceeding a configurational entropy of 1.5R or by entropy stabilization along one or both axes.
5. Synthesis and Kinetic Effects in High-Entropy Oxides
High-entropy oxides are typically synthesized at high temperatures and retain their high-entropy state through rapid quenching. The synthesis methods and cooling rates significantly affect the final structure and properties of the material. Kinetic factors in the formation of high-entropy materials cannot be ignored, as they determine the phase transitions and structural reorganization of the material during the synthesis process. Different synthesis methods can lead to differences in the microstructure and performance of the material, thus affecting its practical applications. Figures 5 and 6 illustrate the types of local distortions in high-entropy oxides and the structural features due to entropy enhancement from the local environment, as well as the reduction of configurational entropy, emphasizing the impact of synthesis and kinetics on high-entropy oxides. For example, regarding local distortions, the Jahn-Teller effect may cause axial elongation or compression of oxygen octahedra; these distortions, while occurring locally, can have significant impacts on the overall performance of the material. In terms of synthesis and kinetics, studies have shown that rapid quenching can effectively preserve the disordered structure of high-entropy oxides, while slow cooling may lead to aggregation or short-range ordering.
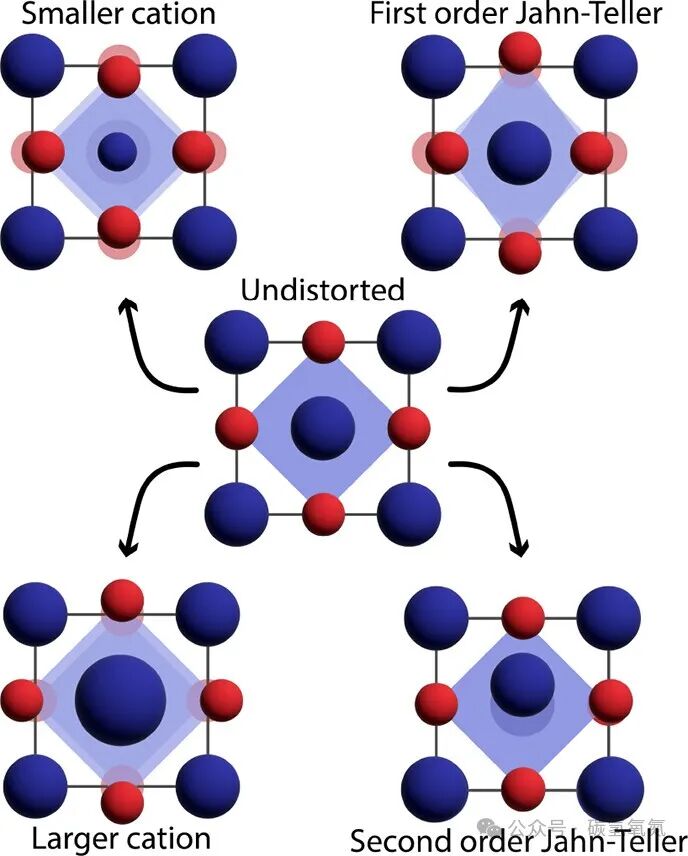
Figure 5: HEOs exhibit a well-defined average undistorted structure (center), but they are susceptible to various local distortions, especially in their oxygen sublattice. Smaller cations (top left) and larger cations (bottom left) can induce isotropic contraction or expansion of coordination polyhedra, while Jahn-Teller active ions (top right) can induce anisotropic distortions in their local environment. Distortions of the cation sublattice, such as cation polar eccentricity caused by so-called second-order Jahn-Teller distortions (bottom right), although less common, can also occur.
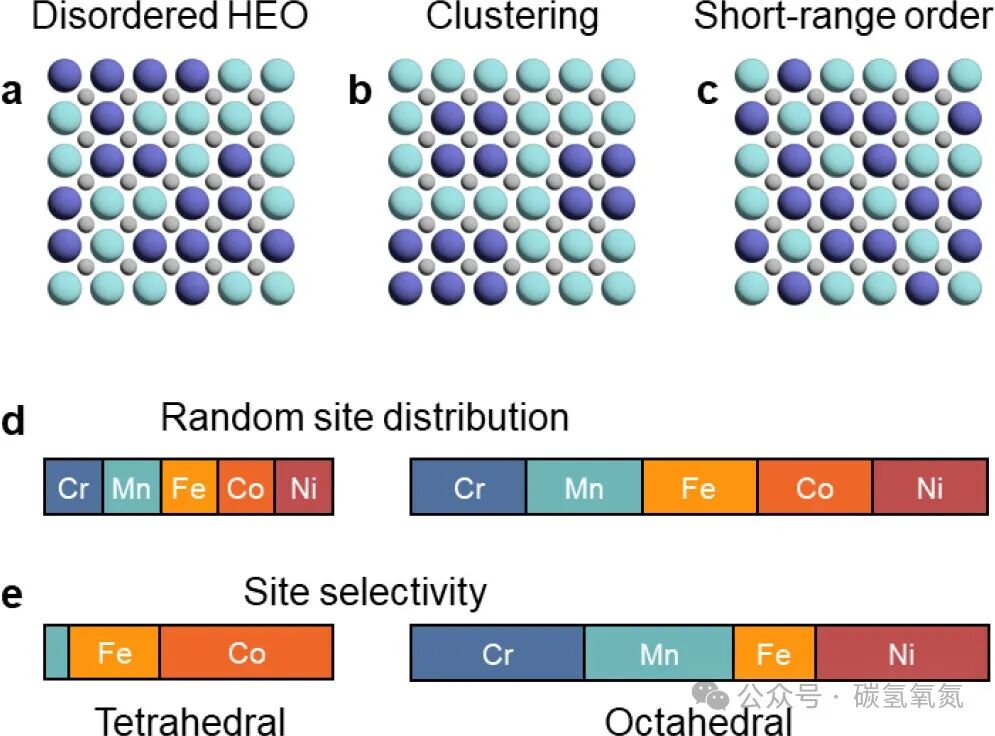
Figure 6: (a) When cations are truly randomly distributed at shared crystallographic sites, HEO achieves ideal configurational entropy. (b) Due to aggregation, cations of the same type tend to be adjacent; or (c) short-range ordering occurs, resulting in partial ordering at small length scales, which may lead to a reduction in configurational entropy. In materials with multiple crystallographically unique cation sites, maximum entropy is achieved through (d) random site distribution, exemplified by octahedral and tetrahedral sites in the 3d transition metal spinel crystal structure, where the number of octahedral sites is twice that of tetrahedral sites. (e) In real materials, crystal field effects dominate over configurational entropy, leading to significant site selectivity.
Figure 7 illustrates the possible structural changes of entropy-stabilized binary compounds under different cooling rates. Rapid cooling can preserve the high-entropy disordered state of the material; slow cooling may lead to long-range ordering or phase separation. Kinetic factors significantly influence the final structure and properties of the material. The phase transition behavior of the material at different cooling rates can be attributed to kinetic competition. At high-temperature equilibrium, the atoms in the material have high kinetic energy, allowing them to move freely and form the thermodynamically most stable phase. When rapidly cooled, atomic diffusion is restricted, preventing the material from reaching thermodynamic equilibrium, thus preserving the high-entropy disordered structure. In contrast, during slow cooling, atoms have sufficient time to diffuse and reorganize, potentially forming long-range ordered phases or undergoing phase separation. This kinetic influence depends not only on the cooling rate but also closely relates to the composition, structure, and synthesis methods of the material.
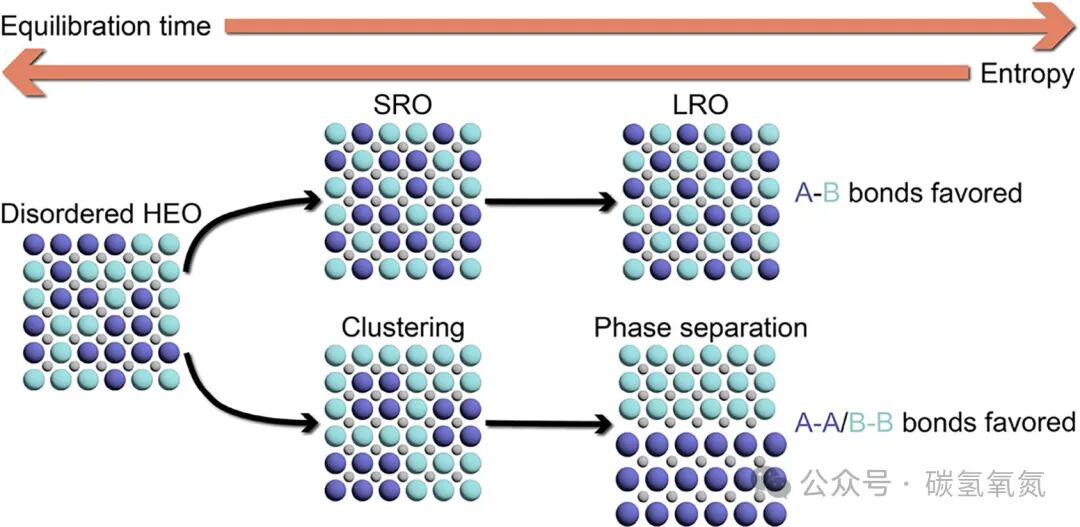
Figure 7: The thermal history experienced by the sample and the energetics of the specific system determine the final state exhibited by the sample. Quenching can preserve high-entropy, high-enthalpy, and completely configurationally disordered HEO. Extending the equilibrium time at high temperatures allows for the formation of long-range order (LRO) or complete phase separation. Intermediate equilibrium times may lead to aggregation or short-range order (SRO).
6. Outlook
Despite the progress made in the study of high-entropy oxides, many issues remain to be explored in depth. For example, how to measure and calculate configurational entropy more accurately, and how to better consider the effects of local environment and kinetic factors in theoretical models. Furthermore, the application potential of high-entropy oxides in various fields remains to be further explored, such as in catalysis, sensors, and energy storage materials. Future research should focus on these issues to promote further development in the field of high-entropy oxides. In the computational aspect of high-entropy materials, current research mainly focuses on how to efficiently predict and design new high-entropy materials. For instance, methods such as cluster expansion and machine learning interatomic potentials (MLIPs) can effectively simulate and predict the structure and properties of high-entropy materials. These methods, combined with experimental techniques such as atom probe tomography and extended X-ray absorption fine structure analysis, help to more accurately understand and quantify entropy in high-entropy materials.
References: Aamlid S S, Oudah M, Rottler J, et al. Understanding the Role of Entropy in High Entropy Oxides[J]. Journal of the American Chemical Society, 2023, 145 (12): 5991 − 6006. DOI:10.1021/jacs.2c11608.
—————————————————————————————Creating content is not easy, please click on the bottom advertisement before leaving. 