
Click the blue text to follow us!
From December 7 to 10, 2021, the 44th San Antonio Breast Cancer Symposium (SABCS) was grandly held. At this conference, updated follow-up data from the TEXT and SOFT studies over 13 years [1] were presented, along with the results of four randomized controlled trials (ABCSG 12, TEXT, SOFT, and HOBOE), involving a total of 7030 female participants [2]. The results showed that ovarian function suppression (OFS) combined with aromatase inhibitors (AI) continues to provide greater benefits than OFS combined with tamoxifen (TAM). Professor Wang Shu from Peking University People’s Hospital provides an in-depth interpretation of these two studies, explaining the choices for adjuvant endocrine therapy in premenopausal hormone receptor-positive early breast cancer patients from a clinical scholar’s perspective.
 Professor Wang Shu
Professor Wang Shu
MD, Chief Physician, currently the Director of the Breast Center at Peking University People’s Hospital, Doctoral Supervisor, Standing Committee Member of the Breast Cancer Professional Committee of the Chinese Anti-Cancer Association (CBCSG), Standing Committee Member of the Breast Cancer Professional Committee of the Chinese Society of Clinical Oncology (CSCO-BC), Committee Member of the Breast Group of the Surgery Branch of the Chinese Medical Association, Vice Chairman of the Breast Disease Professional Committee of the Chinese Medical Education Association, Vice Chairman of the Breast Disease Expert Committee of the Beijing Medical Association, and Vice Chairman of the Breast Surgery Professional Committee of the Beijing Oncology Society.
Research Details
Randomized controlled study comparing adjuvant AI Exemestane (E) + OFS versus TAM + OFS in HR+ early premenopausal breast cancer patients: Update of SOFT-TEXT study (Abstract No: GS2-05)[1]
Research Background
At a median follow-up of 9 years, the combined analysis of SOFT-TEXT showed that adjuvant E + OFS significantly improved disease-free survival (DFS) and distant recurrence-free interval (DRFI) in premenopausal HR+ early breast cancer patients compared to TAM + OFS, although no differences were observed in overall survival (OS) between the two groups. Given the high OS rates in both groups and the long-term recurrence risk of HR+ BC, continued follow-up is crucial for assessing treatment benefits; thus, the conference updated the 13-year follow-up results of the SOFT-TEXT study.
Research Methods
From November 2003 to April 2011, the TEXT and SOFT studies enrolled 5738 premenopausal HR+ early breast cancer patients (2672 in the TEXT study and 3066 in the SOFT study) (Figure 1).
The primary endpoint was defined as DFS, which includes invasive local, regional, distant recurrence, contralateral breast cancer, second malignancies, and death. Secondary endpoints included invasive breast cancer-free interval (BCFI), DRFI, and OS.
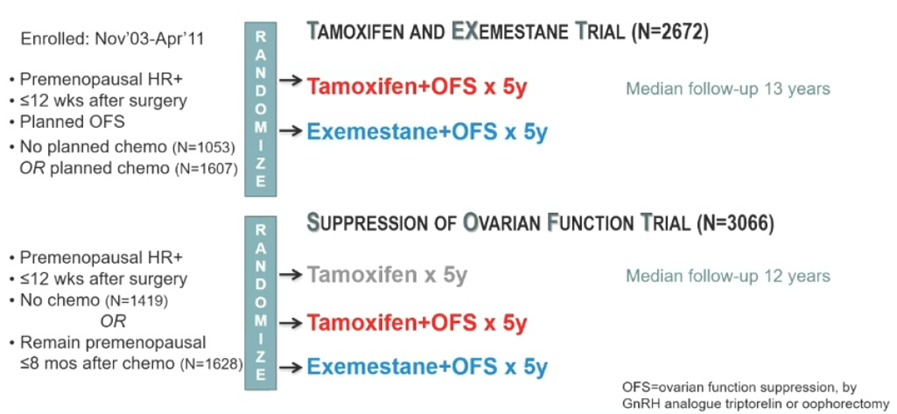
Figure 1. Study Design
Research Results
In the overall analysis of SOFT, with a median follow-up of 12 years, the 12-year DRFI rates for OFS + E compared to OFS + TAM and TAM were 87.8%, 86.2%, and 84.8% respectively (Figure 2.1); as of the database lock, a total of 4690 patients were included in the analysis, with 953 DFS events and 473 deaths occurring in both groups. In the ITT population, compared to TAM + OFS (n=2344), patients in the E + OFS group (n=2346) showed sustained significant improvements in DFS, BCFI, and DRFI outcomes. The 12-year DRFI improved by 1.8%. The 12-year OS rates for the two groups were 90.1% vs 89.1% (HR 0.93; 95% CI, 0.78-1.11) (Figure 2.2).
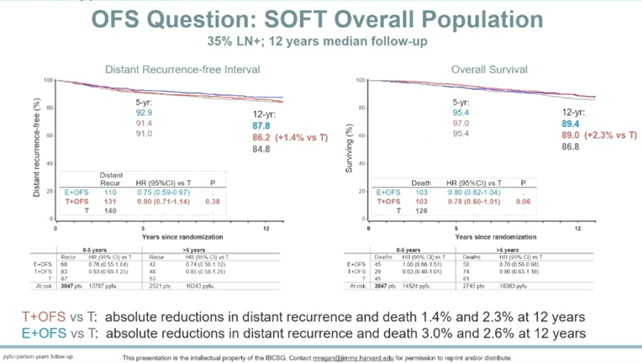
Figure 2.1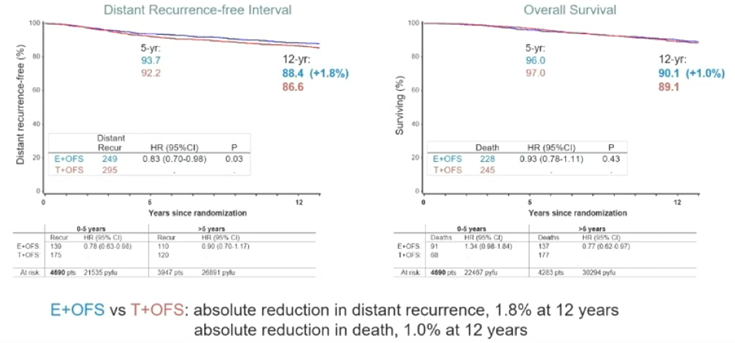
Figure 2.2 DRFI and OS Risks
Research Conclusion
After 13 years of follow-up, adjuvant E + OFS significantly reduced the recurrence risk of HR+ early breast cancer compared to TAM + OFS, with more pronounced benefits in HER2- patients and those at high risk of recurrence, such as those requiring adjuvant chemotherapy or with G3 tumors. Clinically, appropriate patients should be selected for OFS treatment. The follow-up of this study will continue for another 5 years to assess the risks of distant recurrence and death.
Meta-analysis comparing AI versus TAM combined with OFS treatment in premenopausal ER+ early breast cancer patients (Abstract No: GS2-04)[2]
Research Background
For hormone receptor (HR)+ early breast cancer patients, TAM adjuvant therapy can reduce the risk of breast cancer mortality by one-third over 15 years. In postmenopausal patients, the efficacy of AI is superior to that of TAM; however, for premenopausal patients, due to compensatory ovarian estrogen production, AI is ineffective when used alone. Previously, several clinical studies have evaluated whether AI combined with OFS is more effective than TAM in preventing recurrence of breast cancer in premenopausal women, but the results varied.
Research Methods
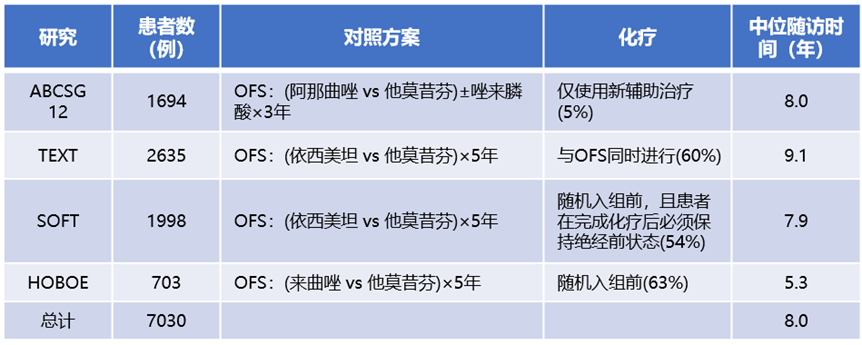
This meta-analysis published by EBCTCG included data from four randomized controlled trials involving 7030 premenopausal ER+ breast cancer patients. All patients who underwent medical or surgical castration were randomly assigned to receive AI or TAM treatment for 3 years (ABCSG XII study) or 5 years (SOFT, TEXT, and HOBOE studies). The primary endpoint of the meta-analysis was the recurrence of invasive breast cancer (distant, local regional, or new contralateral primary) and breast cancer mortality. Log-rank analysis was used to assess the relative risk (RR) and confidence intervals (CIs) of the first event, with the distribution of the four studies shown in Table 1.
Table 1. Distribution of the Four Studies
Research Results
The meta-analysis showed that compared to OFS + TAM, women receiving OFS + AI treatment had an average annual recurrence rate reduced by 21% (RR 0.79, 95% 0.69-0.90, p=0.0005) (Figure 3).
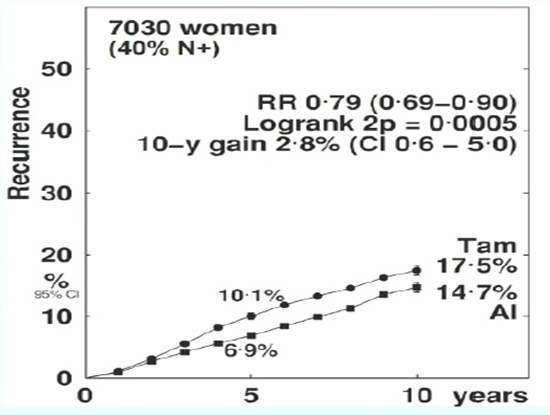
Figure 3. Recurrence Risk
The study found that the main benefits of AI occurred in the first 1-4 years (RR 0.68, 99% CI 0.58-0.80), while the treatment differences between the two groups were not significant in years 5-9 (RR 0.98, 99% 0.73-1.32) (Figure 4). Additionally, the absolute risk of distant recurrence of breast cancer in the AI group was reduced by 1.9% compared to the TAM group (12.1% vs 10.2%, p<0.05). OFS combined with AI reduced the risk of distant recurrence by 17% (RR 0.83, 95% CI 0.71-0.97, p=0.02), but there was no difference in breast cancer mortality (Figure 5).
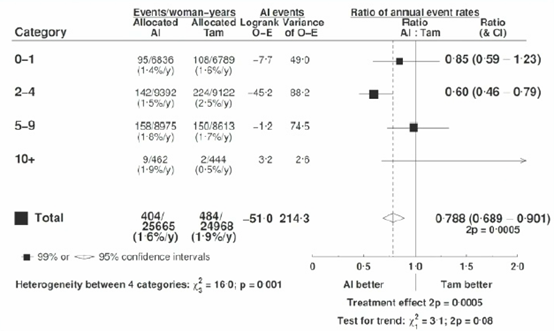
Figure 4. Recurrence Risk at Different Follow-up Times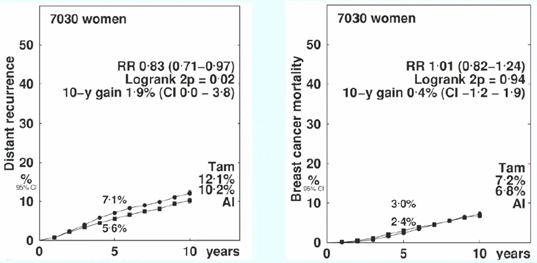
Figure 5. Distant Recurrence and Mortality Risks
Subgroup analysis showed that the reduction in recurrence rates during different treatment periods was not affected by age, BMI, tumor size, grade, histological subtype, or whether chemotherapy was administered. Regardless of lymph node status (N0 or N1-3), AI significantly reduced the risk of breast cancer recurrence compared to TAM (N0: 6.8% vs 9.0%, RR 0.71, 95% CI 0.54-0.95; N1-3: 13.3% vs 17.3%, RR 0.71, 95% CI 0.53-0.96) (Figure 6).
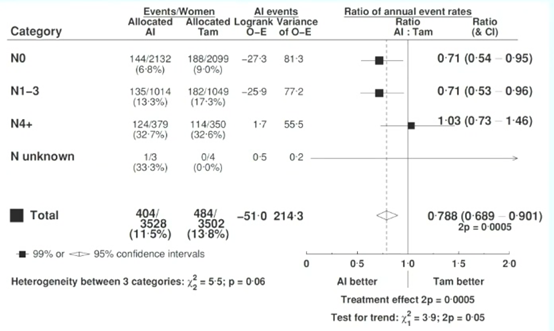
Figure 6. Analysis of Recurrence and Lymph Node Status Correlation
There were no significant differences in non-breast cancer mortality risks between the two groups. In terms of safety, patients receiving AI treatment had a higher incidence of fractures (5.0% vs 3.8%, p=0.02).
Research Conclusion
In premenopausal women, combining AI with OFS reduces the breast cancer recurrence risk by approximately 21% compared to TAM. For premenopausal ER+ patients with intermediate to high recurrence risk, OFS + AI may provide greater absolute benefits than OFS + TAM treatment.
Research Commentary
Breast cancer is the most prevalent malignant tumor among women worldwide, with approximately 50-60% of HR-positive early breast cancer patients in China [3]. Endocrine therapy is the primary means of reducing the recurrence risk in these patients. The SOFT-TEXT study is one of the key studies establishing the role of OFS in adjuvant endocrine therapy for HR+ early breast cancer, and its long-term follow-up and overall survival data over 10 years have significant clinical reference value. Additionally, high-quality meta-analyses provide a higher level of evidence. These two studies prompt us to consider two aspects.
1. Efficacy and Recurrence Risk of Endocrine Therapy in HR+ Early Breast Cancer Patients.
From the results of these two studies, the SOFT-TEXT study data show that the 5-year DFS rate for OFS + AI is 91.1%, the 8-year DFS rate is 86.8%, and the 12-year DFS rate is 85.9%, indicating that HR+ early patients receiving OFS + AI adjuvant therapy have good survival benefits. However, we also observe that as time progresses, the recurrence risk for these patients gradually increases, necessitating our attention. The results of the meta-analysis published by EBCTCG are consistent with those of the SOFT-TEXT study. The analysis of recurrence risk and follow-up years among the overall population shows that OFS + AI reduces the recurrence risk by approximately 21% (RR value of 0.79), which is statistically significant; in the analysis of the correlation between follow-up years and recurrence, the recurrence in the AI group was significantly lower than that in the TAM group during years 2-4, indicating a relatively significant benefit of OFS + AI treatment. However, the treatment advantage of combined AI was not significantly different from that of combined TAM in years 5-9; the reasons for the differences in data results between the two groups remain unclear, and may relate to the heterogeneity of the data itself, the risk levels of different populations, and the long-term efficacy differences of various treatment regimens. Regardless, subgroup data should be considered as a reference.
2. Clinical Choices for Adjuvant Therapy in HR+ Early Breast Cancer.
In clinical practice, the treatment of early breast cancer patients primarily considers two aspects: short-term efficacy and long-term benefits. We screen patients for sensitive treatment regimens through neoadjuvant therapy based on their pCR status; we achieve long-term benefits through comprehensive management and planning of the patient’s treatment regimen. Different treatment regimens need to be matched with suitable patient populations. From the long-term follow-up results of SOFT-TEXT at 5, 8, and 12 years, it is evident that OFS + AI adjuvant therapy provides consistent survival benefits for early HR+ breast cancer patients, indicating clear clinical benefits for early intermediate to high-risk HR-positive patients receiving OFS combined with AI adjuvant therapy. Furthermore, CDK4/6 inhibitors also play an important role in endocrine therapy for breast cancer. Preclinical data suggest that dual inhibition of CDK4/6 and ER signaling has a synergistic effect and can inhibit the growth of G1 phase ER+ breast cancer cells. However, there may be differences between different CDK4/6 inhibitors. The MonarchE [4] study showed that the Abemaciclib + standard endocrine therapy group had significant iDFS benefits compared to the single-agent endocrine therapy group in the adjuvant treatment of high-risk early HR-positive breast cancer patients; however, the PALLAS [5] and PENELOPE-B [6] studies did not observe significant benefits of Palbociclib in early adjuvant treatment. In addition, new SERD drugs such as Amcenestrant and Giledestrant (RG6171) are also undergoing research for the treatment of HR+ patients. At this conference, we also saw Academician Xu Binghe present data from a Phase III clinical study of the HDAC inhibitor Entinostat combined with Exemestane for HR+ advanced breast cancer [7], which exerts its effects by enhancing sensitivity to endocrine therapy. We look forward to these new drugs that enhance endocrine therapy or reverse endocrine therapy resistance providing more new data in early treatment.
The road to breast cancer treatment is long and arduous, but with the continuous emergence of large-scale evidence, the publication of long-term follow-up data, and the ongoing development of new treatment drugs, we can expect a brighter future!
References
[1] Randomized comparison of adjuvant aromatase inhibitor exemestane (E) plus ovarian function suppression (OFS) vs tamoxifen (T) plus OFS in premenopausal women with hormone receptor-positive (HR+) early breast cancer (BC): update of the combined TEXT and SOFT trials. SABCS 2021 abs GS2-05.
[2] Bradley R, et al. Aromatase inhibitors versus tamoxifen in pre-menopausal women with estrogen receptor positive early stage breast cancer treated with ovarian suppression: A patient level meta-analysis of 7,030 women in four randomized trials. SABCS 2021 abs GS2-04.
[3] Expert Consensus on the Clinical Application of Ovarian Function Suppression in Early Breast Cancer in China (2018). Chinese Journal of Cancer. 2018, 28(11): 871-880.
[4] Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol. 2020 Dec 1;38(34):3987-3998.
[5] Tan Y, Lima Neto F, Braga-Neto U. et al. PALLAS: Penalized mAximum LikeLihood and pArticle Swarms for Inference of Gene Regulatory Networks from Time Series Data. IEEE/ACM Trans Comput Biol Bioinform. 2020 Nov 10;PP.
[6] Loibl S, Marmé F, Martin M, Untch M, et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer-The Penelope-B Trial. J Clin Oncol. 2021 May 10;39(14):1518-1530.
[7] Binghe Xu, et al. A Randomized Controlled Phase III Trial of Entinostat, a Class I Selective Histone Deacetylase Inhibitor, in Combination with Exemestane in Patients with Hormone Receptor Positive Advanced Breast Cancer. SABCS 2021 abs GS1-06.
Source: Oncology News
Disclaimer
All content in this article is sourced from the internet, and we maintain neutrality regarding the viewpoints expressed. We do not provide any explicit or implicit guarantees regarding the accuracy, reliability, or completeness of the content included, and we are not responsible for the opinions expressed in the article. Copyright belongs to the original author.