One patient had all tumor target lesions disappear, achieving complete remission,80% of patients experienced significant tumor reduction, and the novel GCC19CAR-T brings new hope to patients with advanced colorectal cancer!
Globally, colorectal cancer (CRC) has become the third most common malignant tumor and the second leading cause of cancer-related deaths. Currently, metastatic colorectal cancer primarily relies on chemotherapy and targeted therapy, which have shown poor efficacy.
Fortunately, in recent years, new drugs and innovative therapies for colorectal cancer have emerged rapidly. In addition to the well-known surgical, chemotherapy, and targeted therapies, novel treatments such as Yttrium-90, CAR-T, TIL, and other new therapeutic options have made significant breakthroughs, providing opportunities for both early-stage colorectal cancer and late-stage cancer patients who have failed treatment to achieve longer survival through these new approaches.
Recently, at the 2025 Gastrointestinal Cancer Symposium, a highly anticipated Phase 1 clinical trial (NCT05319314) announced exciting preliminary results. The novel CAR-T cell therapy GCC19CAR-T has demonstrated exceptional clinical anti-tumor activity in treating refractory metastatic colorectal cancer (mCRC), bringing new hope to patients in treatment dilemmas.
Objective response rate reaches 80%! GCC19CAR-T brings new hope to patients with advanced colorectal cancer
Guanylate cyclase 2C (GUCY2C, GCC) plays a key role in the fluid and ion homeostasis of the gastrointestinal tract, with approximately 80% of colorectal cancer patients expressing GCC in their tumors, which is considered a “golden” target that can precisely attack colorectal cancer cells!
Based on this, researchers developed the CAR-T cell therapy targeting GCC, GCC19CAR-T. Currently, GCC19CAR-T has been tested on 35 patients in a trial approved by the IRB in China, and the trial data has been presented at numerous conferences such as ASCO, ASGCT, and AACR, causing a huge sensation!
Data from a recent clinical trial published in JAMA Oncology in September 2024 shows that out of 15 patients, 9 (60%) were female, with a median age (range) of 44 years (33-61 years). The objective response rate was 40%, with 2 out of 8 patients receiving treatment with 1×10^6 cells/kg or 2×10^6 cells/kg achieving partial remission; among 7 patients receiving 2×10^6 cells/kg treatment, 4 achieved partial remission. At the time of data cutoff, the median overall survival was 22.8 months; the median progression-free survival in the high-dose group was 6.0 months.
Additionally, the literature reported 3 cases of partial remission after treatment, which is very encouraging.
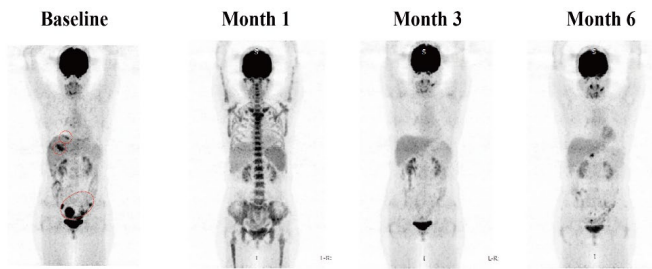
Significant reduction in abdominal and pelvic target lesions, achieving partial remission
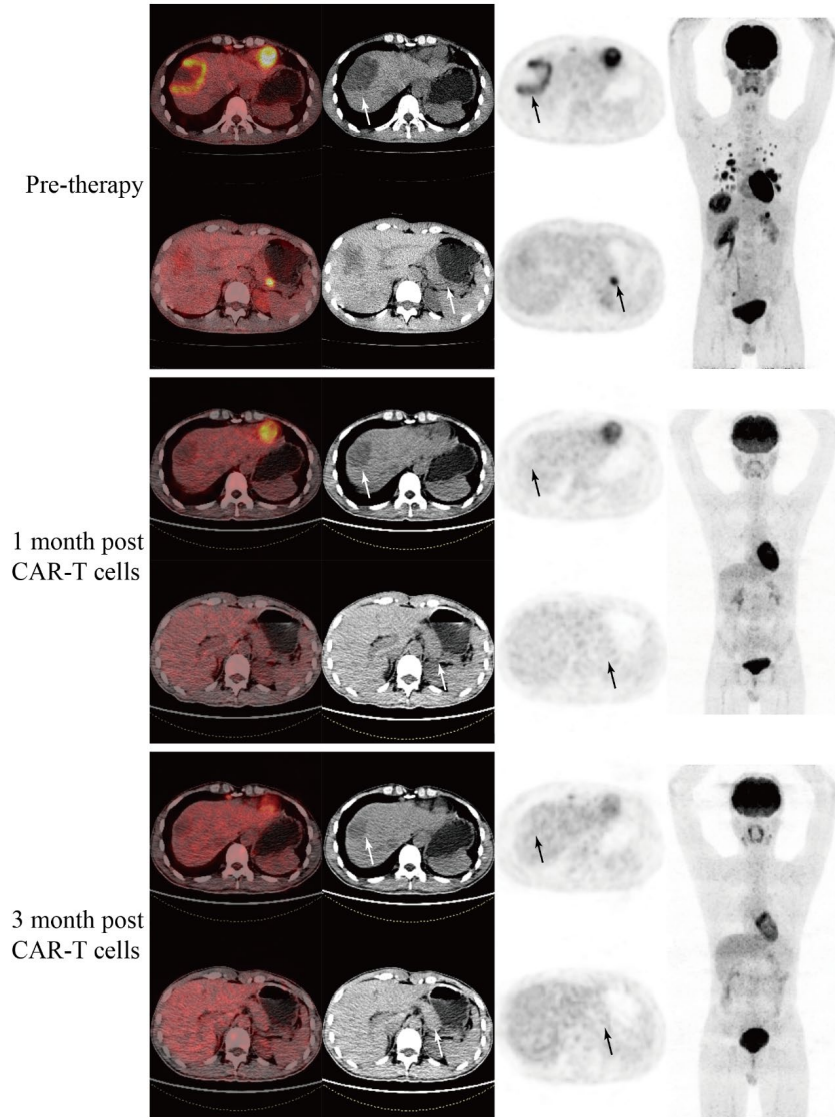 Three months post-treatment, the metastatic lesions (including liver and pancreas) showed no FDG uptake, achieving partial remission
Three months post-treatment, the metastatic lesions (including liver and pancreas) showed no FDG uptake, achieving partial remission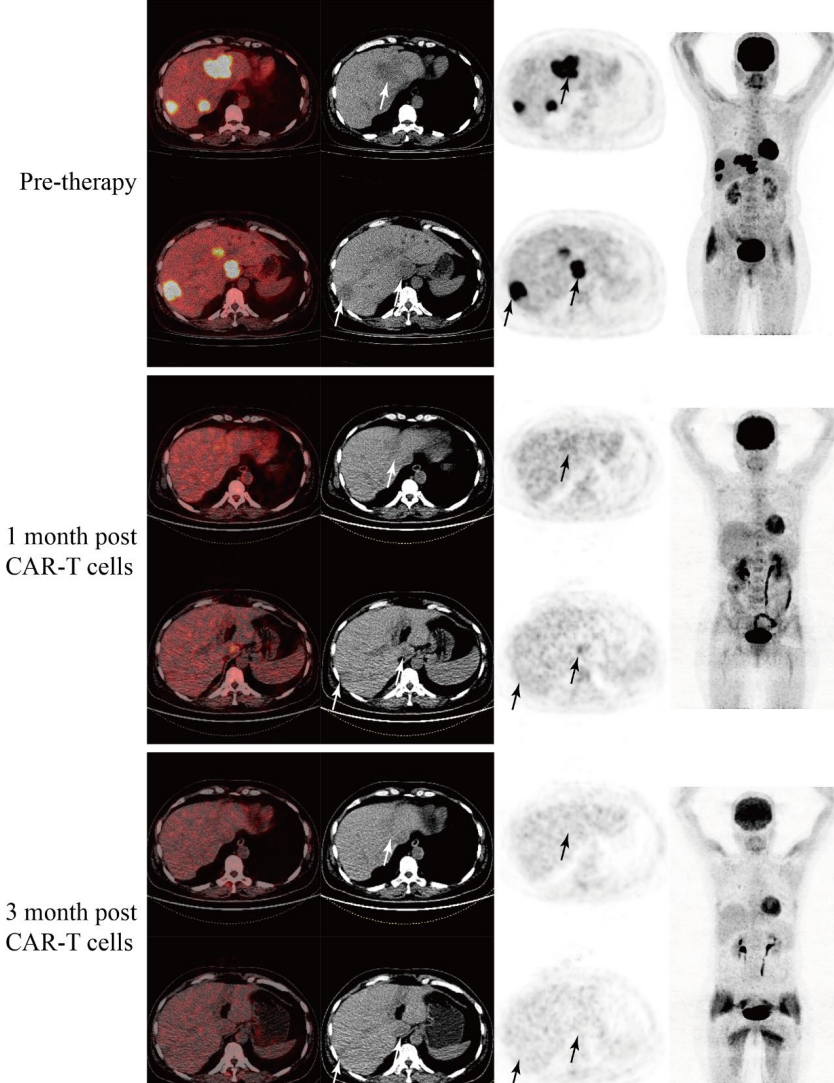 Three months post-treatment, the volume of liver metastases significantly reduced, and the liver lesions showed no FDG uptake, achieving partial remissionLatest research results presented at the 2025 Gastrointestinal Cancer Symposium showed that researchers evaluated two different doses of GCC19CAR-T. In dose group 1 (1 x 10⁶ cells/kg), 4 evaluable patients were included, with an overall response rate (ORR) of 25.0%, where 1 patient achieved confirmed partial remission (PR), and 2 patients showed partial metabolic remission with stable disease (SD); while the performance in dose group 2 (2 x 10⁶ cells/kg) was even more impressive, with an ORR of 80.0% among 5 patients, where one fortunate patient had all tumor target lesions disappear, achieving pathological complete remission! Three lesions significantly reduced, achieving partial remission, and another patient showed complete metabolic remission in PET/CT examination. Overall, across both dose levels (9 patients in total), the ORR reached 56%. The disease control rate was 75% in dose group 1 and 80% in dose group 2, with an overall disease control rate of 78%.
Three months post-treatment, the volume of liver metastases significantly reduced, and the liver lesions showed no FDG uptake, achieving partial remissionLatest research results presented at the 2025 Gastrointestinal Cancer Symposium showed that researchers evaluated two different doses of GCC19CAR-T. In dose group 1 (1 x 10⁶ cells/kg), 4 evaluable patients were included, with an overall response rate (ORR) of 25.0%, where 1 patient achieved confirmed partial remission (PR), and 2 patients showed partial metabolic remission with stable disease (SD); while the performance in dose group 2 (2 x 10⁶ cells/kg) was even more impressive, with an ORR of 80.0% among 5 patients, where one fortunate patient had all tumor target lesions disappear, achieving pathological complete remission! Three lesions significantly reduced, achieving partial remission, and another patient showed complete metabolic remission in PET/CT examination. Overall, across both dose levels (9 patients in total), the ORR reached 56%. The disease control rate was 75% in dose group 1 and 80% in dose group 2, with an overall disease control rate of 78%.
“These preliminary results fully demonstrate that GCC19CAR-T has significant clinical anti-tumor activity in patients with refractory metastatic colorectal cancer. Although the trial is still ongoing, the current results are already highly significant, and we look forward to further updates,” emphasized Dr. Bridget Keenan and her team, the principal authors of the study from the Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco, during the poster presentation.
However, while observing the positive effects, attention must also be paid to treatment-related adverse reactions. Data shows that all patients experienced cytokine release syndrome (CRS). The team is actively exploring optimization strategies to better manage treatment-related diarrhea and colitis.”
Good news! Multiple cancer centers in China have initiated clinical trials for CAR-T cell therapyThe CAR-T therapy in the aforementioned study is still in the clinical trial stage for solid tumors, but with the evolution of CAR-T generations, there have been significant improvements in proliferation, cytokine release, and other aspects, and many clinical trials have applied CAR-T for the treatment of solid tumors, with some fortunate patients achieving complete remission, bringing a warm spring to patients with advanced solid tumors!These studies hope to bring new hope to patients. The good news is that these cutting-edge T therapies are currently recruiting various solid tumor patients for clinical trials, seekingCAR-T, TCR-T, TIL and other cell therapies and new treatment technologies from both domestic and international sources, and those who can afford it can apply through the following process.Application Process
1. Submit medical records to the Global Oncology Doctors Network Medical Department at 4006667998 as required;
2. The medical department will conduct a preliminary assessment and match suitable clinical trials;
3. Submit medical records to the corresponding trial center;
4. After evaluation, assist patients in participating in clinical trials.
Additionally, several CAR-T therapies targeting the following solid tumor sites are currently undergoing clinical trials in China:
 Types of cancers undergoing CAR-T clinical trials
Types of cancers undergoing CAR-T clinical trials
Claudin18.2: forgastric cancer, pancreatic cancer and others;
Mesothelin: for treatingmesothelioma, pancreatic cancer, ovarian cancer, lung cancer;
CEA: for treatinglung cancer, colorectal cancer, gastric cancer, breast cancer, and pancreatic cancer;
MUC-1: for treatingliver cancer, lung cancer, pancreatic cancer, colorectal cancer, gastric cancer;
GPC3: for treatingliver cancer;
GUCY2C: forcolorectal cancer;
EGFRvII: for treatingglioma, head and neck tumors;
B7-H3: for treatingrhabdomyosarcoma, Wilms tumor, neuroblastoma, medulloblastoma, and particularly difficult-to-treat brainstem tumors (DIPG);
PSMA: forprostate cancer and others.
If patients with the above cancers wish to test relevant targets or learn more details, they can call the Global Oncology Doctors Network Medical Department for evaluation.
Before the emergence of cell therapies, solid tumors, including advanced gastric adenocarcinoma and pancreatic cancer, were typically treated with surgery, radiotherapy, and chemotherapy, with gastric adenocarcinoma accounting for 95% of gastric malignancies, and pancreatic cancer being one of the most malignant tumors with a median survival and 5-year survival rate far below other tumors, known as the “king of cancers.” However, most patients experience local recurrence or metastasis after surgery, and these malignant tumors are also not very sensitive to radiotherapy and chemotherapy. Therefore, based on current standard therapies, treatment outcomes are not ideal, and prognosis is extremely poor. The emergence of immunotherapy will bring long-term survival hope and miracles to more advanced patients.
We firmly believe that with the establishment of a comprehensive regulatory system for cell immunotherapy, the country will open the doors to cell immunotherapy, benefiting more cancer patients, and our country’s cell immunotherapy will also step onto the international stage!This article is an original work of the Global Oncology Doctors Network and is strictly prohibited from being reproduced without authorization.
Scan to add to the patient group
Anti-cancer information | New technologies | New drug development | Authoritative experts
Join the group to receive anti-cancer materials for free

 Cell Immunotherapy
Cell Immunotherapy








