Bioorthogonal chemistry provides powerful tools for the selective labeling of biomolecules within complex biological systems, greatly advancing research in areas such as complex cellular processes, protein dynamics, and protein interactions. However, the development of bioorthogonal chemistry still faces numerous challenges. In February 2025, Dr. Dominik Schauenburg and Professor Tanja Weil from the Max Planck Institute for Polymer Research in Germany published a forward-looking article titled “Not So Bioorthogonal Chemistry” in the J. Am. Chem. Soc., discussing the “non-bioorthogonal” aspects of reactions commonly referred to as “bioorthogonal”.
In practical applications, bioorthogonal chemical reactions must meet the following criteria: (1) they should have good biocompatibility, allowing for mild reactions under physiological conditions and at the corresponding temperatures, without the use of organic solvents; (2) they should exhibit orthogonal reaction characteristics and high chemical selectivity, acting only on one functional group of biomolecules in the presence of numerous reactive groups; (3) they should have fast reaction kinetics to ensure effective reactions. However, when two or more bioorthogonal reactions occur simultaneously, various unwanted side reactions such as covalent bond loss and reverse reactions may arise, posing significant challenges to chemical selectivity.
The authors first introduce the potential side reactions and kinetic effects of the bioorthogonal reaction between azides and cyclooctynes. As shown in Figures 1A and B, organic azides can undergo Staudinger ligation with phosphines to form amide bonds, while also reacting with cyclooctyne in a [3+2] cycloaddition reaction. By comparing the second-order rate constants, it is evident that the [3+2] cycloaddition reaction dominates under kinetic control (10-2 −100 M-1 s-1 > 10-4 −10-2 M-1 s-1), primarily due to the ring strain of cyclooctyne. However, when the concentration of cyclooctyne is low or the reaction time is prolonged, the Staudinger ligation can also occur, yielding amide products. In previous studies by Professor Bertozzi, it was even demonstrated that the Staudinger ligation reaction could proceed normally with an excess of azide. For example, the reactions of cyclooctyne compounds with azides and tetrazoles (Figures 1C and D) show that the second-order rate constant for the [3+2] cycloaddition reaction with azides is significantly lower than that for the inverse electron-demand Diels−Alder reaction with tetrazoles. These examples suggest that when selecting bio-conjugation reactions, it is essential to fully consider kinetic factors and the reaction mechanisms of various reactions under complex conditions to achieve the desired chemical selectivity.
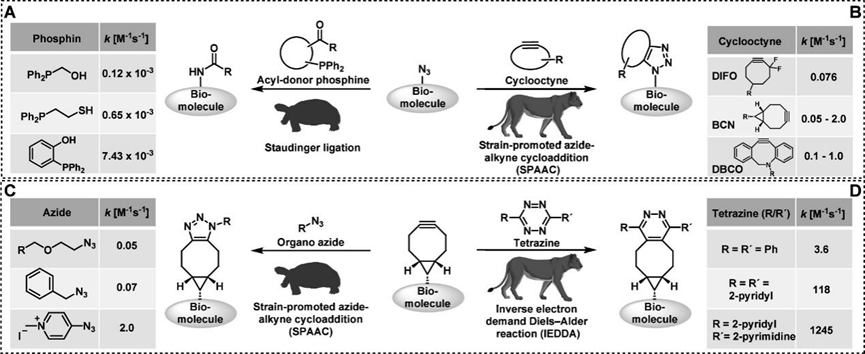 Figure 1 Illustration of reactions involving azides and cyclooctynes
Figure 1 Illustration of reactions involving azides and cyclooctynes
Next, the authors primarily discuss the potential unexpected side reactions in bio-conjugation reactions using the Michael addition reaction of thiols as an example. The selective coupling between thiols and maleimides has been utilized in various fields, including drug development, but the side reactions that occur cannot be overlooked. As shown in Figure 2, when conducting bio-conjugation under basic conditions, particularly at pH > 7.5, maleimides can undergo nucleophilic addition with amines (such as protein N-termini, lysine side chains, etc.), providing potential off-target reaction pathways. Additionally, in the presence of common reducing agents like TCEP, it can also undergo Michael addition with maleimides to generate phosphonium betaine structures; electron-deficient azide groups can also react with electron-rich maleimide groups to form stable five-membered triazolines through [3+2] cycloaddition. The existence of these side reactions indicates that when employing multiple bioorthogonal reactions simultaneously, one should consider the potential side reactions between various substances and precisely control experimental conditions to avoid cross-talk between reactions.
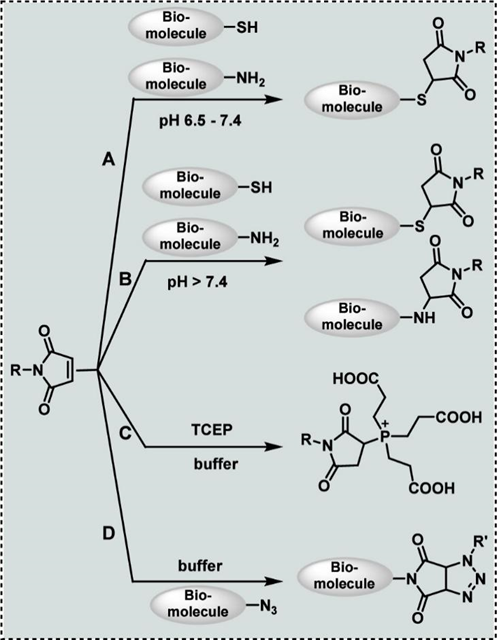 Figure 2 Potential side reactions of maleimide groupsSubsequently, the authors discuss potential side reactions and the “non-bioorthogonal” implications from three aspects: metal-catalyzed bio-conjugation reactions, photochemical bioorthogonal reactions, and the bioorthogonal chemistry of dyes, with specific examples including Cu(I) catalyzed reactions of azides with alkynes, photoinitiated thiol-ene and thiol-yne reactions, and the addition reactions of Cy5 dye with reducing agents like TCEP.Finally, the authors summarize and prospect the discussion results, stating that the development of bioorthogonal chemistry still faces challenges, including the reversibility of reactions, unexpected side reactions, and the complexities introduced by catalysts and photochemical activation. In future developments, it is essential to continuously develop new bioorthogonal strategies to improve chemical selectivity, reaction efficiency, and stability, especially in complex biological environments such as living cells, to gain a deeper understanding of both expected and unexpected reaction mechanisms and to develop better reaction strategies.Editor: Zhou WeishenReviewer: Zhou Keting
Figure 2 Potential side reactions of maleimide groupsSubsequently, the authors discuss potential side reactions and the “non-bioorthogonal” implications from three aspects: metal-catalyzed bio-conjugation reactions, photochemical bioorthogonal reactions, and the bioorthogonal chemistry of dyes, with specific examples including Cu(I) catalyzed reactions of azides with alkynes, photoinitiated thiol-ene and thiol-yne reactions, and the addition reactions of Cy5 dye with reducing agents like TCEP.Finally, the authors summarize and prospect the discussion results, stating that the development of bioorthogonal chemistry still faces challenges, including the reversibility of reactions, unexpected side reactions, and the complexities introduced by catalysts and photochemical activation. In future developments, it is essential to continuously develop new bioorthogonal strategies to improve chemical selectivity, reaction efficiency, and stability, especially in complex biological environments such as living cells, to gain a deeper understanding of both expected and unexpected reaction mechanisms and to develop better reaction strategies.Editor: Zhou WeishenReviewer: Zhou Keting
Author Information
Dr. Dominik SchauenburgTenure-track Assistant Professor, Department of Instructive Biomaterials Engineering, MERLN Institute for Technology-Inspired Regenerative Medicine, Maastricht University.His research group operates at the interface of chemistry, biology, and materials science, developing molecular strategies to engineer dynamic systems that adapt, respond, and evolve—just like nature intended.Prof. Dr. Tanja WeilProfessor, Max Planck Institute for Polymer Research.Her group develops innovative synthesis concepts for the production of functional macromolecules and hybrid materials through complex molecular design strategies to solve future challenges in biomedicine and materials science.