Due to WeChat’s changes to subscription account push rules, many friends cannot quickly find us, but we are actually pushing every day!You can set Organic Synthesis as a star (click the upper right “…” to set it as a star), so you can quickly find us in the WeChat subscription account and check the daily subscriptions.
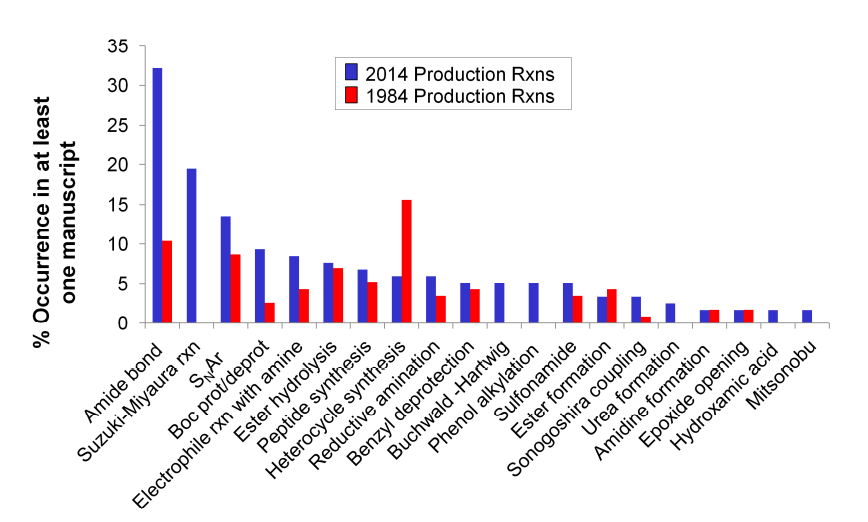
In 2016, Dean G. Brown and others published an article in the J. Med. Chem. journal that statistically analyzed the frequency of various types of chemical reactions from 1984 to 2014 (125 articles from J. Med. Chem. were selected from both 1984 and 2014). They compared the trends in the frequency of various reactions over 20 years, and the top five reaction types in 2014 were: (1) Amide bond formation, (2) SNAr reactions, (3) Boc protection/deprotection reactions, (4) Ester hydrolysis reactions, (5) Suzuki−Miyaura coupling.[J. Med. Chem. 2016, 59, 4443−4458]
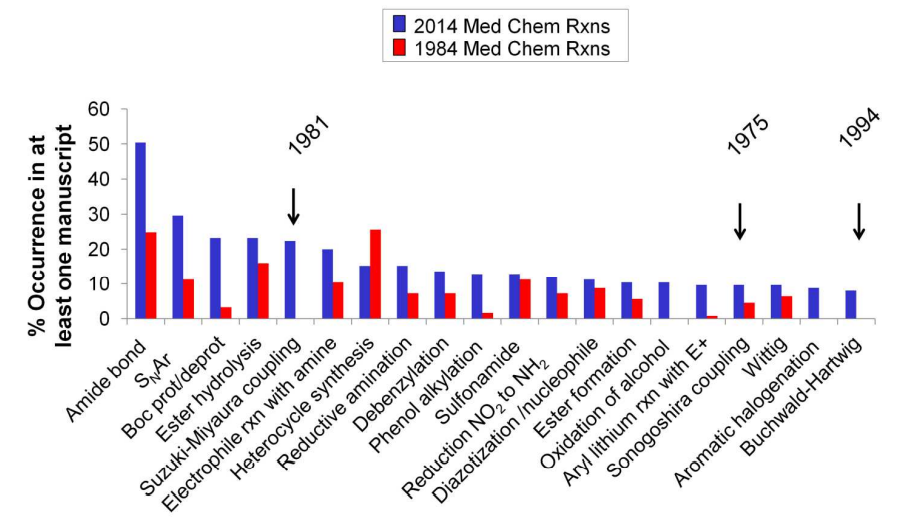
The ranking of the frequency of various reaction types in the final step shows that the Suzuki–Miyaura reaction ranks second, indicating its importance in drug synthesis.
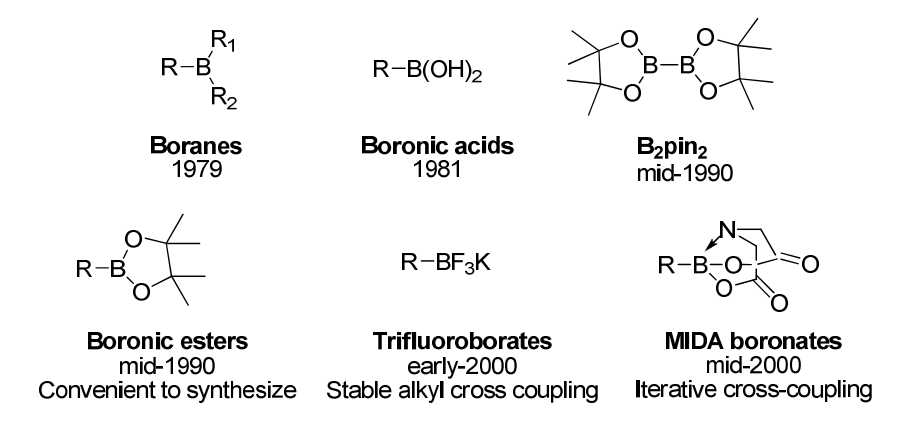
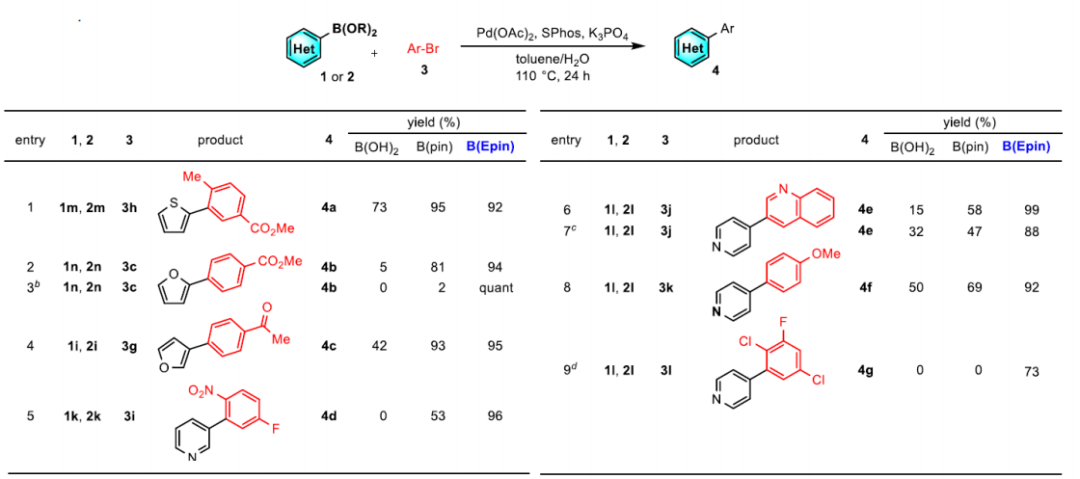
Due to its high application frequency, organic boron reagents used for reactions are also continuously developing. The latest discovery is the Burke boronic acid reagent found in 2007. However, since pinacol boronic esters are easy to synthesize, they have the widest application range. But those who have worked with boronic esters know that during reaction monitoring, the system is very clean, but after column chromatography purification, a portion of the product is likely to be converted into boronic acid, and additionally pinacol boronic esters tend to tail during column chromatography, making it very difficult to obtain a highly pure boronic ester.[Burke boronic acid reagent]
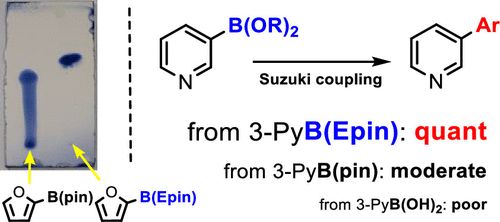
Recently, Naoki Oka and others reported a new type of aryl boronic ester—-aryl boronic acid 1,1,2,2-tetraethyl ethylene glycol ester [ArB(Epin)s]. This type of boronic ester is very suitable for purification using silica gel column chromatography, and the separation yield is very high, overcoming the decomposition and tailing problems of conventional boronic esters. Utilizing ArB(Epin) for Suzuki–Miyaura reactions also yields better results than the corresponding aryl boronic acids or aryl boronic acid pinacol esters.[Org. Lett. 2022, 24, 19, 3510–3514]
This reagent can be synthesized similarly to pinacol boronic esters and is very convenient to synthesize. It can be obtained by esterification of aryl boronic acid with 1,1,2,2-tetraethyl ethylene glycol, or by using B2Epin2 for Miyaura boronic esterification reactions.
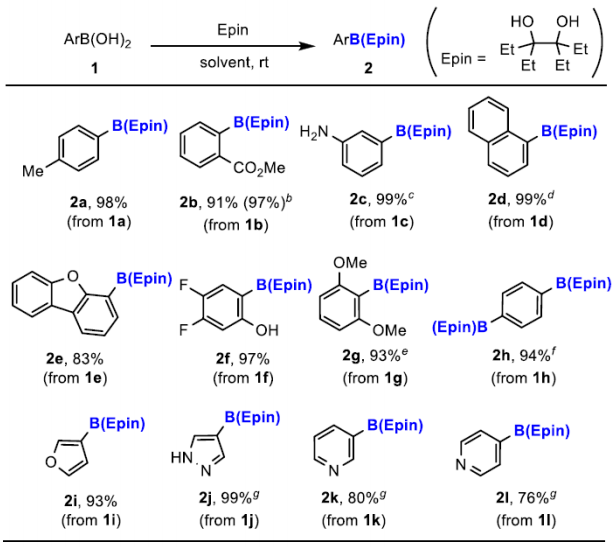
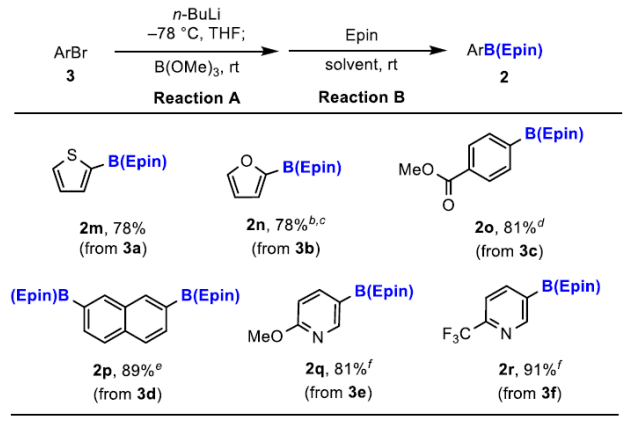
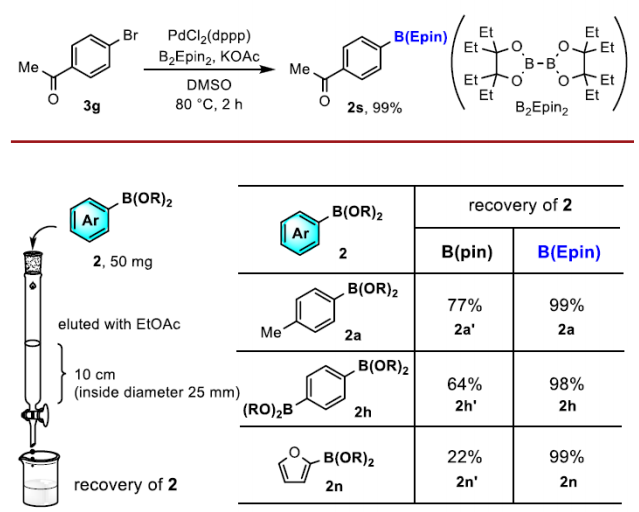
Compared to pinacol boronic esters, this reagent has the advantage of being stable on silica gel and very easy to separate.
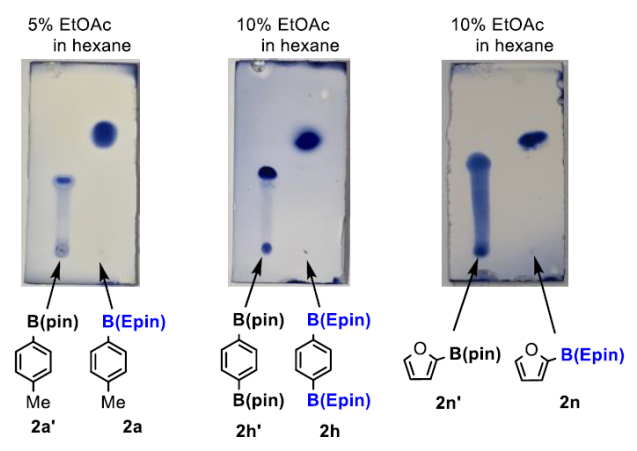
Comparing the activity of the Suzuki–Miyaura reaction, ArB(Epin) also outperforms the corresponding aryl boronic acids or aryl boronic acid pinacol esters.
Reaction Operations
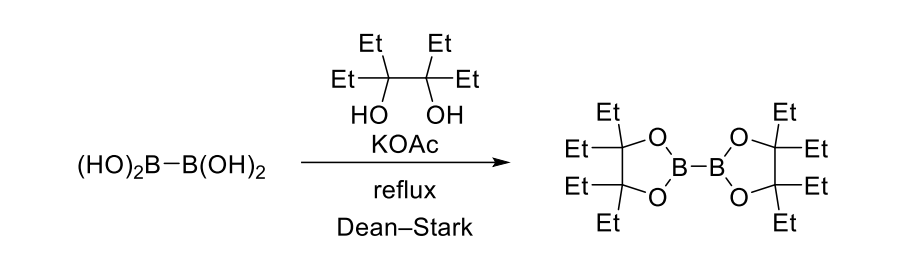
A 100 mL flask was charged with tetrahydroxydiboron 0.38 g (4.2 mmol), Ethylpinacol 1.5 g (8.4 mmol) and potassium acetate 1.0 g (11 mmol) under N2 atmosphere, then it was diluted with toluene 40 mL, and refluxed for 16 h with a Dean-Stark apparatus. The reaction mixture was cooled to room temperature, filtered through Celite, concentrated under reduced pressure to gain 4,4,4’,4’,5,5,5’,5’-octaethyl-2,2’-bis(1,3,2-dioxabororan)[5a] as a colorless solid (1.5 g, 99%). Mp 62.7–64.5 °C. 1H NMR (300 MHz, CDCl3) δ: 1.75–1.60 (16 H, m), 0.90 (24 H, t, J = 7.5 Hz). 13C NMR (100 MHz, CDCl3) δ: 88.3, 26.4, 8.9.
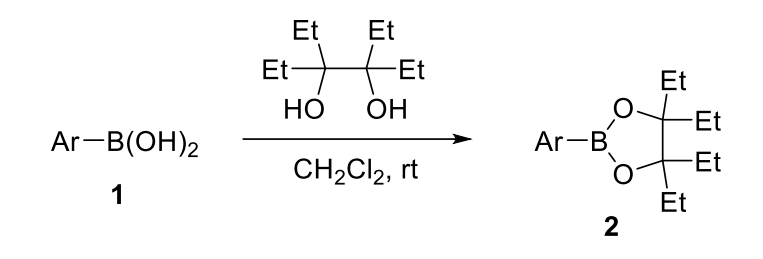
General Procedure I: A flask with a magnetic stir bar was charged with boronic acid 1 (1.0 equiv) and 3,4-diethylhexane-3,4-diol, Epin (1.0 equiv) and evacuated and back-filled with nitrogen. Anhydrous CH2Cl2 (0.10 M) was added to the flask via a syringe, and the mixture was stirred for 16 h at room temperature. After the reaction was quenched with H2O, the reaction mixture was extracted thrice with CH2Cl2. The combined organic phase was dried over anhydrous MgSO4 and the solvent was removed under reduced pressure. The crude product was purified by flash column chromatography on silica gel (a mixture of hexane and EtOAc) to afford aryl boronic acid 3,4-diethylhexane-3,4-diol ester, ArB(Epin) 2.
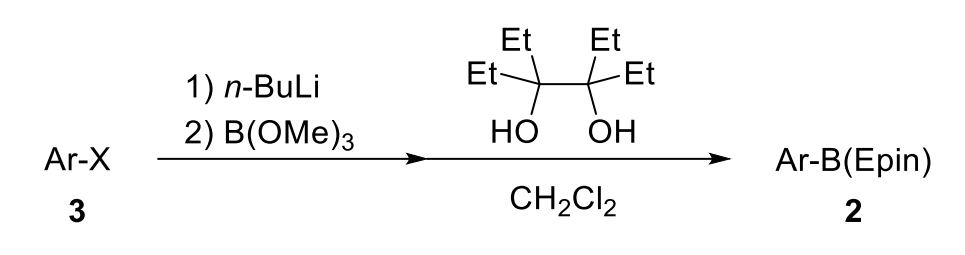
General Procedure II: An oven-dried flask with a magnetic stir bar equipped with an inlet adapter with three-way stopcock was evacuated and back-filled with nitrogen. Aryl halide 3 (1.0 equiv) was charged in the flask and evacuated and back-filled with nitrogen. Anhydrous THF (0.10 M) was added to the flask via a syringe and cooled to –78 °C. 2.7 M n-BuLi in hexane [or 1.0 M i-PrMgCl·LiCl in THF or 1.6 M t-BuLi in pentane] (1.2 equiv) was added to the mixture at –78 °C via a syringe and the mixture was stirred for 1 h. After B(OMe)3 (2.0 equiv) was added to the reaction mixture, the reaction mixture was allowed to warm up to room temperature and stirred for 3 h. A saturated solution of NH4Cl was added to the mixture, and the mixture was extracted thrice with EtOAc. The combined organic phase was washed with brine. The organic phase was dried over MgSO4, and the solvent was removed under reduced pressure. The flask was charged with 3,4-diethylhexane-3,4-diol, Epin (1.0 equiv) in CH2Cl2 (10 mL, 0.10 M), and stirred at room temperature. The mixture was evaporated under reduced pressure. The residue was purified by column chromatography on silica gel (a mixture of hexane and EtOAc) to afford aryl boronic acid 3,4-diethylhexane-3,4-diol ester, ArB(Epin) 2.
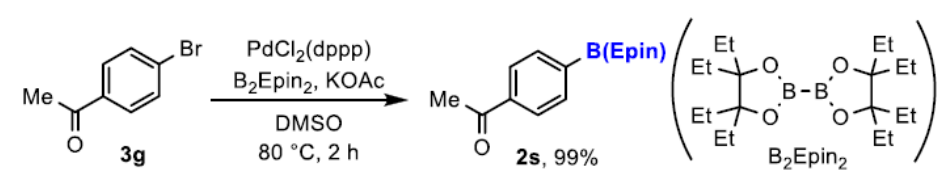
An oven-dried Schlenk flask was charged with PdCl2(dppp)·CH2Cl2 (2.9 mg, 5.0 mol%), KOAc (29 mg, 0.30 mmol), B2(Epin)2 [1] (55 mg, 0.15 mmol) and a magnetic stirrer bar. The flask was equipped with a rubber septum and evacuated and back-filled with argon (this process was repeated three times). After 1-(4-bromophenyl)ethan-1-one (3g) (20 mg, 0.10 mmol) in anhydrous DMSO S-14 (1.0 mL, 0.10 M) was added via a syringe, the septum was replaced with a Teflon screw cap under a flow of argon, and the cap was tightly closed. The mixture was stirred for 2 h at 80 °C. After cooling to room temperature, the mixture was filtered through a short pad of celite. The filtrate was evaporated under reduced pressure. The residue was purified by column chromatography on silica gel (hexane/EtOAc = 95:5) to afford the titled compound 2s as a colorless oil (30 mg, 99%).
References
1. Aryl Boronic Esters Are Stable on Silica Gel and Reactive under Suzuki–Miyaura Coupling Conditions; Org. Lett. 2022, 24, 19, 3510–3514.
2. Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem. 2016, 59, 4443−4458.
