Skip to content
Controlling the global warming temperature rise to 2°C or even 1.5°C is crucial for addressing climate change. Artificially removing carbon dioxide from the atmosphere is key to achieving net-zero CO2 plans. Carbon capture and storage (CCS) plays an important role in reducing emissions associated with fossil fuel use.Direct air capture (DAC), which extracts CO2 from the air through artificial contactors, has very high potential. However, DAC is a relatively novel technology that is still in the early stages of development and commercialization, with many issues yet to be resolved.
Matteo Gazzani from Utrecht University in the Netherlands recently published a research paper in Joule, providing a technical assessment of three DAC technologies for removing CO2 from the air: two aqueous washing processes and one solid adsorption process. Using simulations and mathematical optimization, productivity, exergy (㶲), and energy consumption were calculated. Additionally, the cost range for large-scale deployment was assessed. The study shows that all technologies can provide high purity CO2, with the solid adsorption-based process having the best potential, as its 㶲 demand is 1.4-3.7 MJkgCO2-1, and productivity is 3.8-10.6 kgCO2:m-3 h-1. Furthermore, the authors demonstrated through a simple model that productivity and energy translate into CO2 capture costs, proving that capital costs are the main cost driver. All technologies have the potential to operate below $200ton-1CO2. When high mass transfer is achieved, and dependency on installation costs is lower, the solid adsorption process can achieve this goal under a broader range of conditions. The research was published under the title “A comparative energy and costs assessment and optimization for direct air capture technologies” in Joule (DOI: 10.1016/j.joule.2021.05.023), with Matteo Gazzani as the corresponding author.
The early DAC consists of two steps.The first step: sodium or potassium hydroxide reacts with carbon dioxide to form soluble carbonates; the second step: the carbonates are causticized with lime (Ca(OH)2), producing calcium carbonate precipitation. However, the most significant issue with early DAC is the inevitably high energy demand for solvent regeneration. A report by the American Physical Society (APS) selected alkaline washing as the benchmark process for DAC, estimating the cost to capture one ton of CO2 at around $600.
Due to the low concentration of CO2 in the atmosphere, it is inevitable to treat a large volume of air to capture the corresponding amount of CO2. Therefore, effective contact with the solvent is crucial. By tailoring absorption units for air capture applications, Holmes and colleagues estimated that the total cost of air contactors alone could be reduced to $60/ton CO2. However, the major drawbacks associated with the caustic recovery of alkaline hydroxides remain unaddressed.
Custelcean et al. described another washing-based DAC process. In this process, CO2 is extracted from the air using an amino acid aqueous solution (such as glycine or sarcosine), generating the corresponding carbonates. The amino acids are then recovered by crystallizing with carbonate anions and guanidine solids. Finally, the carbonate crystals decompose at 60-120°C, releasing high-purity CO2. Compared to alkaline washing processes, the solvent regeneration temperature is lower, but the process energy consumption is higher.
Although amine washing is the benchmark technology for post-combustion CO2 capture and has been applied in hundreds of gas separation processes, this liquid washing was not previously classified under DAC. Amine washing has only recently been assessed as DAC. Furthermore, amine washing requires a significant amount of low-grade heat for regeneration, and amines are often corrosive and toxic, degrading easily due to long oxidation times. These drawbacks can be significantly reduced by fixing amines onto solid supports, which can lower the energy needed for regenerating amines. For example, amine-modified porous particles are used in vacuum-pressure temperature swing adsorption (VTSA) cycles to capture CO2. In this process, the unloaded adsorbent contacts the air to capture CO2 at ambient conditions; subsequently, the device is evacuated to a pressure range of 20-400 mbar and heated to 80-130°C to desorb CO2. Finally, the unit is pressurized and cooled to ambient conditions. Metal-organic frameworks (MOFs) can serve as both adsorbents and supports, showing good performance as solid adsorption materials. Their large design space and multifunctionality make them excellent candidates for various gas separation applications; moreover, surface area and pore characteristics can be customized, and open metal sites allow for surface modification with additional functional groups. However, the large-scale production and commercialization of MOFs remain an unresolved challenge. An important issue to consider when dealing with solid adsorbents is the behavior related to water adsorption. Because H2O can affect CO2 adsorption depending on environmental conditions and solid characteristics, it can impact process productivity and energy demand.
Two DAC technologies stand out based on the scale deployed and the level of technology achieved: wet washing with alkaline hydroxide solution and VTSA with supported adsorbents. Additionally, amine washing should also be regarded as a similarly mature DAC technology. These processes have different advantages and disadvantages. Liquid washing is a continuous process that can utilize mature and inexpensive components. However, regeneration is costly and complex, especially for alkaline solvents. The VTSA process is fundamentally simpler because CO2 capture and adsorbent regeneration occur in the same unit. Moreover, the regeneration of the adsorbent occurs at low temperatures. However, this process is not as mature as liquid washing, and the stability of the adsorbent remains an issue, requiring customization and a greater energy demand cycle to achieve high CO2 purity.
The authors provide a thorough process analysis for both aqueous and solid-based DAC technologies in this work. By combining advanced rate-based process modeling with mathematical multi-objective process optimization, specific energy consumption (in MJ kg-1CO2) and CO2 productivity (measured in kgCO2 m-3 h-1) can be calculated.
Process Schemes and Methods
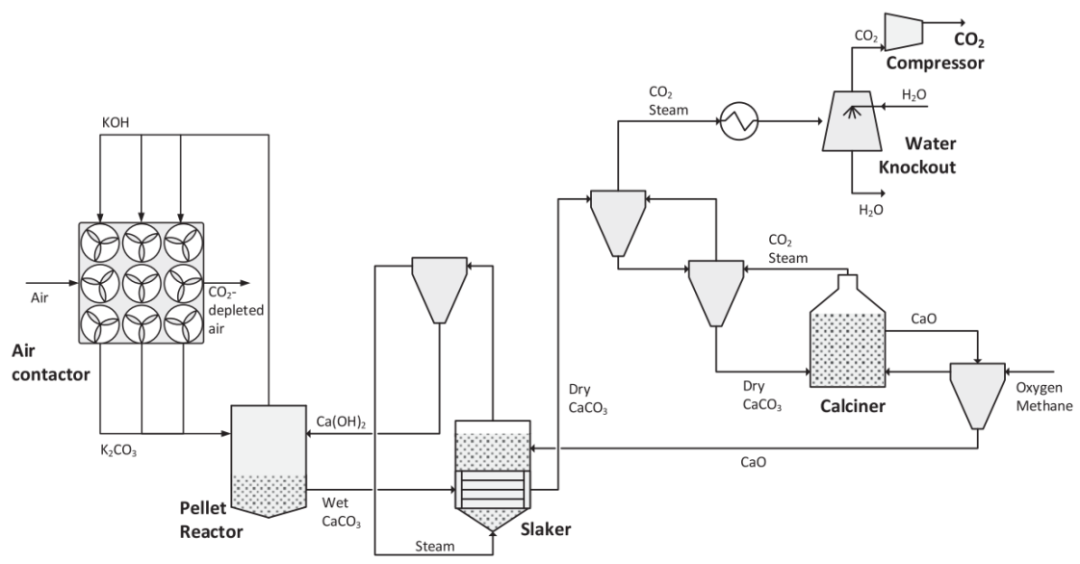
Figure 1: Schematic of the Alkaline Washing DAC Process
The alkaline washing process is illustrated in Figure 1. In alkaline washing, CO2 is captured in a dedicated air contactor unit and absorbed by KOH aqueous solution in the form of K2CO3. The K2CO3 solution is regenerated by producing calcium carbonate, which is sent to a calcination furnace to decompose into CaO and CO2. In this work, the entire process is modeled using Aspen Plus V11, allowing for accurate thermodynamic calculations using the electrolyte NRTL method, while also providing a reliable rate-based model.
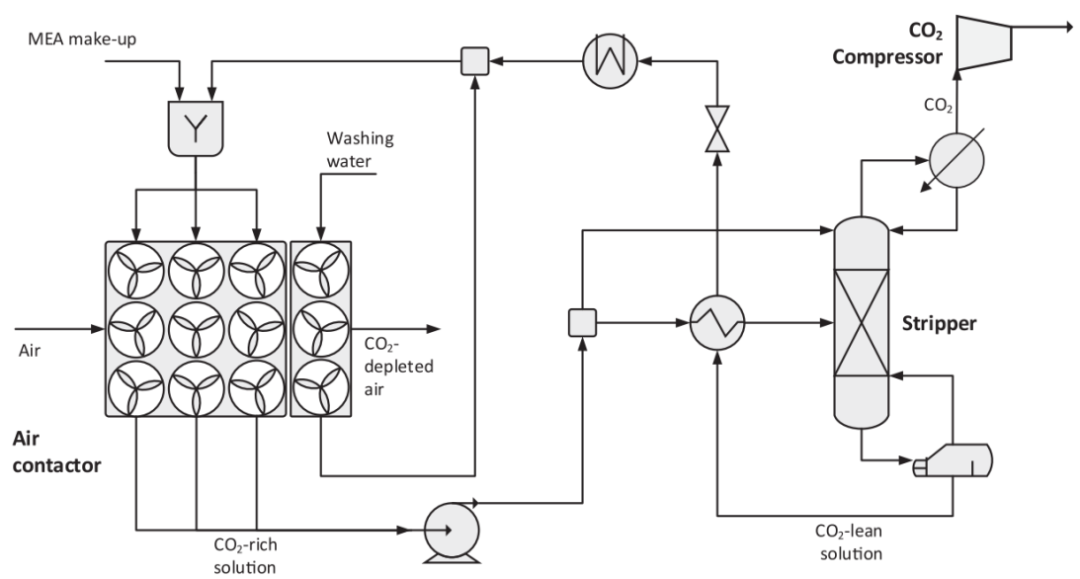
Figure 2: Schematic of the Amine Washing Process
During the CO2 regeneration phase, the amine washing process is much simpler than the alkaline washing. The process layout is shown in Figure 2.
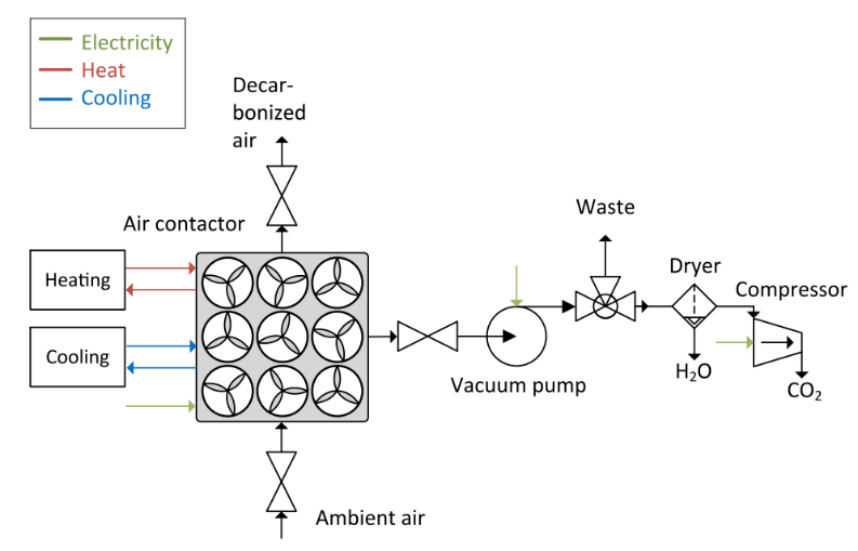
Figure 3: Simplified Flow Process for CO2 Capture Using Solid Adsorption
The simplified solid adsorption process is shown in Figure 3. The unit mainly consists of an air contactor, control valves, vacuum pump, and hot/cold supply system. The solid adsorption process is a cyclic process where individual units sequentially undergo loading (adsorption) and regeneration (desorption) steps (pressure swing adsorption [PSA]) at different pressures. Since DAC treats air under ambient conditions and very high flow rates, compressing air is not feasible, making temperature and vacuum the only regeneration drives. Therefore, the authors simulated the vacuum-temperature swing adsorption (VTSA) four-step cycle process, which includes (i) adsorption, (ii) purging, (iii) regeneration, and (iv) repressurization.
In the adsorption step, the air mixture enters the adsorber unit under ambient conditions. CO2 (and H2O) is selectively removed by the adsorbent, and the air leaving the system contains trace amounts of CO2. This step terminates when the front of CO2 reaches the end of the fixed bed. To improve CO2 purity, a preliminary heating step is introduced to remove the air in the void space (mainly N2). The adsorbent is heated to temperature T1<T2 by external heating fluid, where 1 represents preheating and 2 represents the heating step. Simultaneously, a vacuum is generated using a vacuum pump. At this step, a small amount of CO2 and H2O has already been desorbed. In the main desorption step, the adsorbent is heated to the maximum working temperature T2 while maintaining vacuum conditions. At this step, high-purity CO2 is generated in water vapor and extracted from the tower. Compared to aqueous-based systems, this is sufficient to regenerate the adsorbent at 100°C. Subsequently, the valve at the inlet is opened, allowing ambient air to flow in, cooling the adsorbent and repressurizing the system until the chromatographic column returns to its initial state.
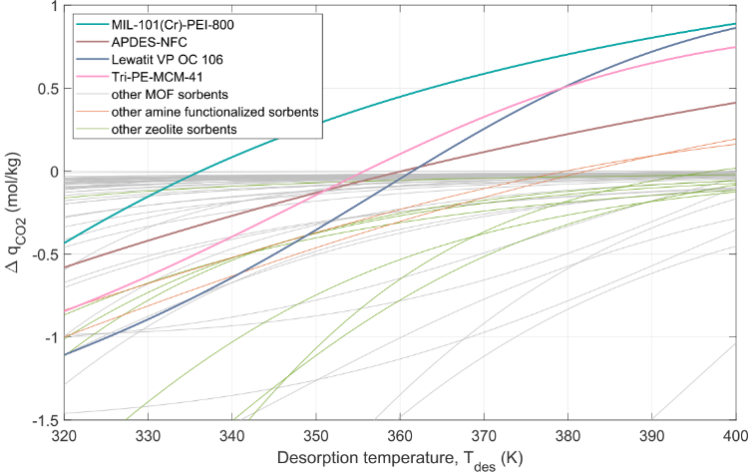 Figure 5: Working Capacities of Various Solid Adsorbents, Including Zeolites (Green Line), MOFs (Gray Line), and Amines (Orange Line)
Multi-Objective Optimization
To determine the optimal performance and related operational variables of different capture systems, the authors conducted multi-objective optimization. It includes two competing objectives: productivity and the electrical and thermal energy consumption coupled with exergy in mass ratio, which is the operational cost.
In gas separation, energy and air contactor volume are representative factors of operational and capital costs, respectively. Therefore, using the consistency of energy performance and productivity calculations, the authors mapped the cost of CO2 capture as a function of (i) the cost of air contactor per cubic meter, (ii) electricity price, and (iii) thermal price.
Figure 6A reports the Pareto curve for the KOH washing process. The area below the curve represents the infeasible region, while the area above represents sub-optimal operations. Contrary to productivity, the exergy values at the Pareto front do not change significantly. The demand is primarily determined by oxygen combustion, around 5MJ/kgCO2. Figure 6B shows a breakdown of exergy demands at the two extremes of the Pareto front. Since the calcination process is a high-temperature process, energy and exergy are close to the same value, meaning energy demand is nearly equal to exergy demand. Among them, the energy consumption of the blower varies significantly along the Pareto curve. Additionally, the comparative impact of wind speed is prominent, as both the exergy demand and productivity increase with increasing wind speed. Moreover, the purity of captured CO2 is unrelated to considerations, remaining at 94.7% under dry conditions. The remaining impurities consist of N2, Ar, and O2, depending on the design of the ASU and oxygen combustion chamber. The purity of CO2 from the alkaline washing process can be further enhanced when using gas cleaning processes (such as traditional ccsoxy combustion processes) or by recovering Ar with a third column in ASU.
Figure 5: Working Capacities of Various Solid Adsorbents, Including Zeolites (Green Line), MOFs (Gray Line), and Amines (Orange Line)
Multi-Objective Optimization
To determine the optimal performance and related operational variables of different capture systems, the authors conducted multi-objective optimization. It includes two competing objectives: productivity and the electrical and thermal energy consumption coupled with exergy in mass ratio, which is the operational cost.
In gas separation, energy and air contactor volume are representative factors of operational and capital costs, respectively. Therefore, using the consistency of energy performance and productivity calculations, the authors mapped the cost of CO2 capture as a function of (i) the cost of air contactor per cubic meter, (ii) electricity price, and (iii) thermal price.
Figure 6A reports the Pareto curve for the KOH washing process. The area below the curve represents the infeasible region, while the area above represents sub-optimal operations. Contrary to productivity, the exergy values at the Pareto front do not change significantly. The demand is primarily determined by oxygen combustion, around 5MJ/kgCO2. Figure 6B shows a breakdown of exergy demands at the two extremes of the Pareto front. Since the calcination process is a high-temperature process, energy and exergy are close to the same value, meaning energy demand is nearly equal to exergy demand. Among them, the energy consumption of the blower varies significantly along the Pareto curve. Additionally, the comparative impact of wind speed is prominent, as both the exergy demand and productivity increase with increasing wind speed. Moreover, the purity of captured CO2 is unrelated to considerations, remaining at 94.7% under dry conditions. The remaining impurities consist of N2, Ar, and O2, depending on the design of the ASU and oxygen combustion chamber. The purity of CO2 from the alkaline washing process can be further enhanced when using gas cleaning processes (such as traditional ccsoxy combustion processes) or by recovering Ar with a third column in ASU.
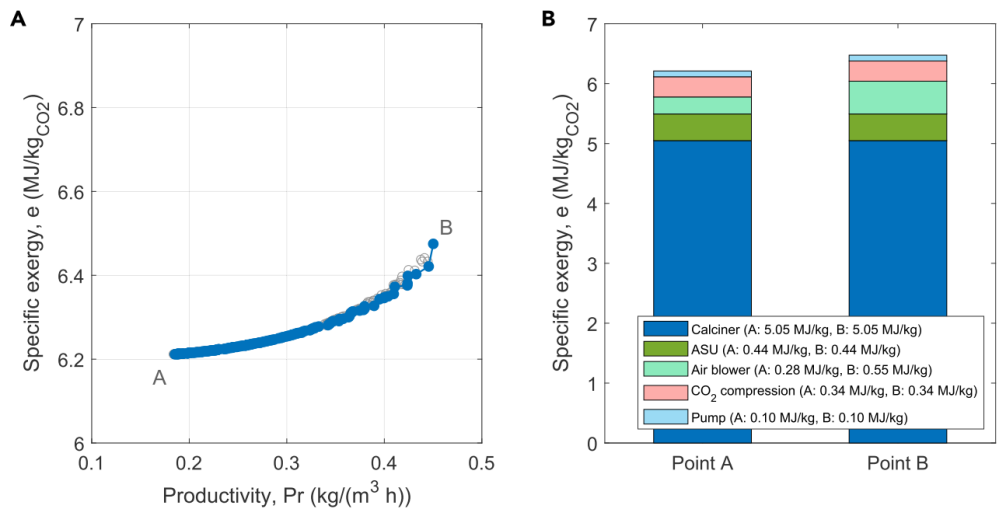 Figure 6: Curves of the KOH Washing Process. (A) Pareto curve of the exergy efficiency of the KOH process, Point A: Minimum exergy consumption; Point B: Maximum productivity. Empty points represent sub-optimal conditions obtained during the optimization process. (B) Breakdown of exergy demands for the two extremes of the Pareto front, namely Points A and B, for the exergy demands of the alkaline washing process. The energy demand equals the exergy demand. Specific values are: calciner A/B: 5.05 MJ/kg, air separation unit A/B: 0.44 MJ/kg, blower A: 0.28 MJ/kg, blower B: 0.55 MJ/kg, CO2 compression A/B: 0.34 MJ/kg, pump A/B: 0.10 MJ/kg.
Amine washing is a mature and widely adopted CO2 capture process. The DAC version described in this work is based on its alkaline counterpart’s unconventional air contactor unit, with solvent regeneration performed through conventional steam stripping. Figure 7A reports the Pareto curve of the amine washing process. It can be seen that both productivity and exergy demand show significant variation along the Pareto curve, highlighting the importance of the optimization process. For the amine washing process, solvent regeneration is the largest contributor to energy demand. Figure 7B shows that for the two extremes of the Pareto front, energy demand is almost entirely caused by the reboiler operation. In other words, higher productivity leads to a dramatic increase in the reboiler’s operating load.
In terms of exergy requirements, the amine and alkaline washing processes are similar, but amine washing requires more energy than alkaline washing. Figure 7B shows the energy demand at Point A of the Pareto, which is the point of lowest energy consumption. Even under these conditions, the energy consumed by the amine washing process is nearly three times that of the alkaline washing process. The breakdown of energy demand at Pareto Points A and B is shown in Figure 7B. Note that the lower energy demand of the blower reported in the figure can be explained by the lower pressure drop provided by the carbon-engineered air contactor.
Figure 6: Curves of the KOH Washing Process. (A) Pareto curve of the exergy efficiency of the KOH process, Point A: Minimum exergy consumption; Point B: Maximum productivity. Empty points represent sub-optimal conditions obtained during the optimization process. (B) Breakdown of exergy demands for the two extremes of the Pareto front, namely Points A and B, for the exergy demands of the alkaline washing process. The energy demand equals the exergy demand. Specific values are: calciner A/B: 5.05 MJ/kg, air separation unit A/B: 0.44 MJ/kg, blower A: 0.28 MJ/kg, blower B: 0.55 MJ/kg, CO2 compression A/B: 0.34 MJ/kg, pump A/B: 0.10 MJ/kg.
Amine washing is a mature and widely adopted CO2 capture process. The DAC version described in this work is based on its alkaline counterpart’s unconventional air contactor unit, with solvent regeneration performed through conventional steam stripping. Figure 7A reports the Pareto curve of the amine washing process. It can be seen that both productivity and exergy demand show significant variation along the Pareto curve, highlighting the importance of the optimization process. For the amine washing process, solvent regeneration is the largest contributor to energy demand. Figure 7B shows that for the two extremes of the Pareto front, energy demand is almost entirely caused by the reboiler operation. In other words, higher productivity leads to a dramatic increase in the reboiler’s operating load.
In terms of exergy requirements, the amine and alkaline washing processes are similar, but amine washing requires more energy than alkaline washing. Figure 7B shows the energy demand at Point A of the Pareto, which is the point of lowest energy consumption. Even under these conditions, the energy consumed by the amine washing process is nearly three times that of the alkaline washing process. The breakdown of energy demand at Pareto Points A and B is shown in Figure 7B. Note that the lower energy demand of the blower reported in the figure can be explained by the lower pressure drop provided by the carbon-engineered air contactor.
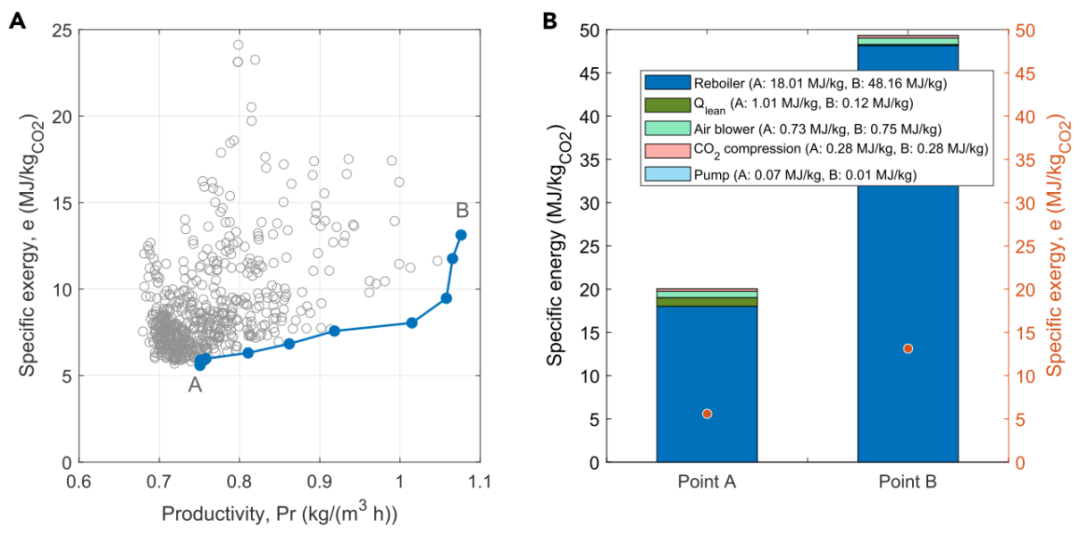 Figure 7: Result Figures of the Amine Washing Process. (A) Pareto front of the exergy efficiency of the amine washing process: Point A: Minimum exergy consumption; Point B: Maximum productivity. Empty points represent sub-optimal conditions. (B) Breakdown of energy demands for the two extremes of the Pareto front, namely Points A and B. Reboiler A: 18.01 MJ/kg, B: 48.16 MJ/kg, lean stream A: 1.01 MJ/kg, B: 0.12 MJ/kg, blower A: 0.73 MJ/kg, B: 0.75 MJ/kg, CO2 compression A/B: 0.28 MJ/kg, pump A: 0.07 MJ/kg, B: 0.01 MJ/kg. The total exergy demand for both points is represented by the orange points, indicated by the right axis.
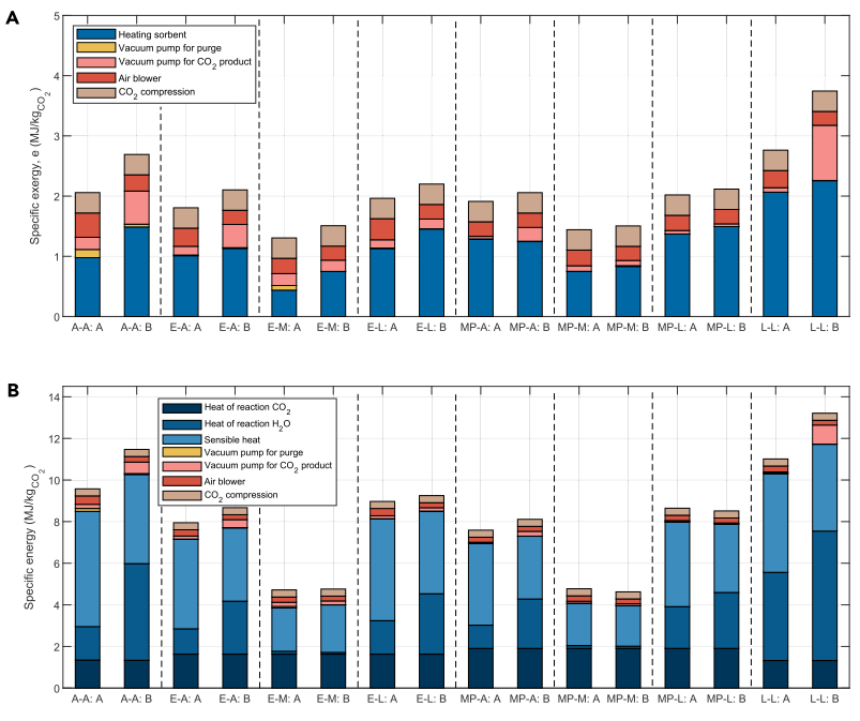
The Pareto curves for different solid adsorbent materials are shown in Figure 8, with specific breakdowns of exergy and energy consumption illustrated in Figure 9. From Figure 8, it can be seen that the solid adsorption process covers a broad range in both exergy (1.5-3.7 MJ/kgCO2) and productivity (3.8-10.6 kg/m3/h). This is due to the differing chemical-physical properties of the adsorbents. The highest exergy consumption is associated with the Lewatit adsorbent. One reason is that the heat of adsorption for chemical adsorption is 91.2 kJ/mol, higher than that of other adsorbents, leading to higher thermal energy demand. The working capacity of water adsorption is also high, further increasing the energy demand during the regeneration phase. Lower particle density and diameter restrict the maximum wind speed, resulting in lower productivity. The MIL-101(Cr)-PEI-800 adsorbent has a high working capacity and low energy consumption, depending on the water isotherm. The MFC-APS-hi water isotherm shows the lowest H2O working capacity, leading to lower exergy consumption for the combinations using this adsorbent (such as MP-M and E-M). For the APDES-NFC adsorbent (A-A), its lower exergy point can compete with the MIL-101(Cr)-PEI-800 adsorbent, but its high productivity is limited to about 6 kg/m3/h. This is due to the adsorbent’s high porosity and low density, along with the lower working capacity of CO2 isotherms, requiring higher regeneration temperatures and lower vacuum pressures during the production process. In all cases, the primary exergy (energy) demand is driven by the heat consumption required during the regeneration process. The energy consumption of the vacuum pump during the purging process is very low, as this step only needs to ensure the removal of all N2 in the void space to achieve high purity, and the process is very quick. The vacuum pump used for the CO2 production step holds a significant share of the total energy consumption, primarily varying with the vacuum pressure required for regeneration. For MP-A, the relatively high vacuum pressure results in very low energy consumption for the pump. The energy consumption of the blower and CO2 compression is similar across all cases. The former has a greater impact on productivity. Due to the lower regeneration temperatures, there is a significant difference between the energy and exergy demands for all solid adsorbents, similar to amine washing (Figure 10).
Figure 7: Result Figures of the Amine Washing Process. (A) Pareto front of the exergy efficiency of the amine washing process: Point A: Minimum exergy consumption; Point B: Maximum productivity. Empty points represent sub-optimal conditions. (B) Breakdown of energy demands for the two extremes of the Pareto front, namely Points A and B. Reboiler A: 18.01 MJ/kg, B: 48.16 MJ/kg, lean stream A: 1.01 MJ/kg, B: 0.12 MJ/kg, blower A: 0.73 MJ/kg, B: 0.75 MJ/kg, CO2 compression A/B: 0.28 MJ/kg, pump A: 0.07 MJ/kg, B: 0.01 MJ/kg. The total exergy demand for both points is represented by the orange points, indicated by the right axis.
The Pareto curves for different solid adsorbent materials are shown in Figure 8, with specific breakdowns of exergy and energy consumption illustrated in Figure 9. From Figure 8, it can be seen that the solid adsorption process covers a broad range in both exergy (1.5-3.7 MJ/kgCO2) and productivity (3.8-10.6 kg/m3/h). This is due to the differing chemical-physical properties of the adsorbents. The highest exergy consumption is associated with the Lewatit adsorbent. One reason is that the heat of adsorption for chemical adsorption is 91.2 kJ/mol, higher than that of other adsorbents, leading to higher thermal energy demand. The working capacity of water adsorption is also high, further increasing the energy demand during the regeneration phase. Lower particle density and diameter restrict the maximum wind speed, resulting in lower productivity. The MIL-101(Cr)-PEI-800 adsorbent has a high working capacity and low energy consumption, depending on the water isotherm. The MFC-APS-hi water isotherm shows the lowest H2O working capacity, leading to lower exergy consumption for the combinations using this adsorbent (such as MP-M and E-M). For the APDES-NFC adsorbent (A-A), its lower exergy point can compete with the MIL-101(Cr)-PEI-800 adsorbent, but its high productivity is limited to about 6 kg/m3/h. This is due to the adsorbent’s high porosity and low density, along with the lower working capacity of CO2 isotherms, requiring higher regeneration temperatures and lower vacuum pressures during the production process. In all cases, the primary exergy (energy) demand is driven by the heat consumption required during the regeneration process. The energy consumption of the vacuum pump during the purging process is very low, as this step only needs to ensure the removal of all N2 in the void space to achieve high purity, and the process is very quick. The vacuum pump used for the CO2 production step holds a significant share of the total energy consumption, primarily varying with the vacuum pressure required for regeneration. For MP-A, the relatively high vacuum pressure results in very low energy consumption for the pump. The energy consumption of the blower and CO2 compression is similar across all cases. The former has a greater impact on productivity. Due to the lower regeneration temperatures, there is a significant difference between the energy and exergy demands for all solid adsorbents, similar to amine washing (Figure 10).
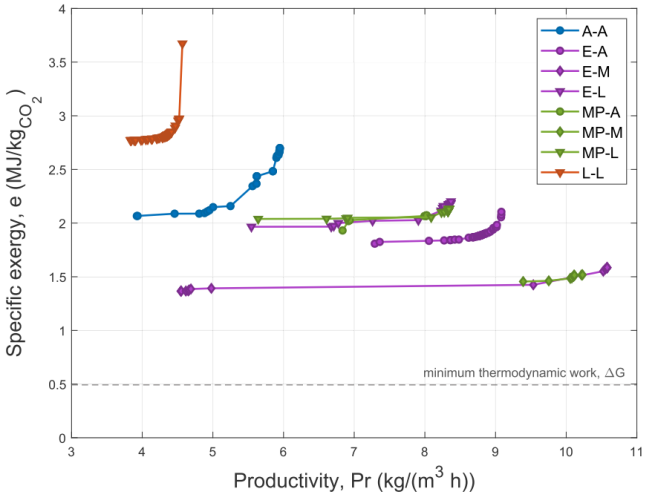 Figure 8: Pareto Curves for Solid Adsorption Optimization Using Various CO2 and H2O Isotherm Combinations
Figure 9: Detailed Energy and Exergy Demands for Different Solid Adsorption Processes. (A) Breakdown of Exergy Demand. (B) Breakdown of Energy Demand. Thermal energy demand is represented in blue, with different shades representing the reaction heat of CO2 and H2O (at equilibrium T=373 K, p = 0.1 bar), as well as the sensible heat of the adsorbent, CO2, and H2O. In both figures, X-X: A represents the minimum exergy/energy on the Pareto, and X-X: B represents maximum productivity.
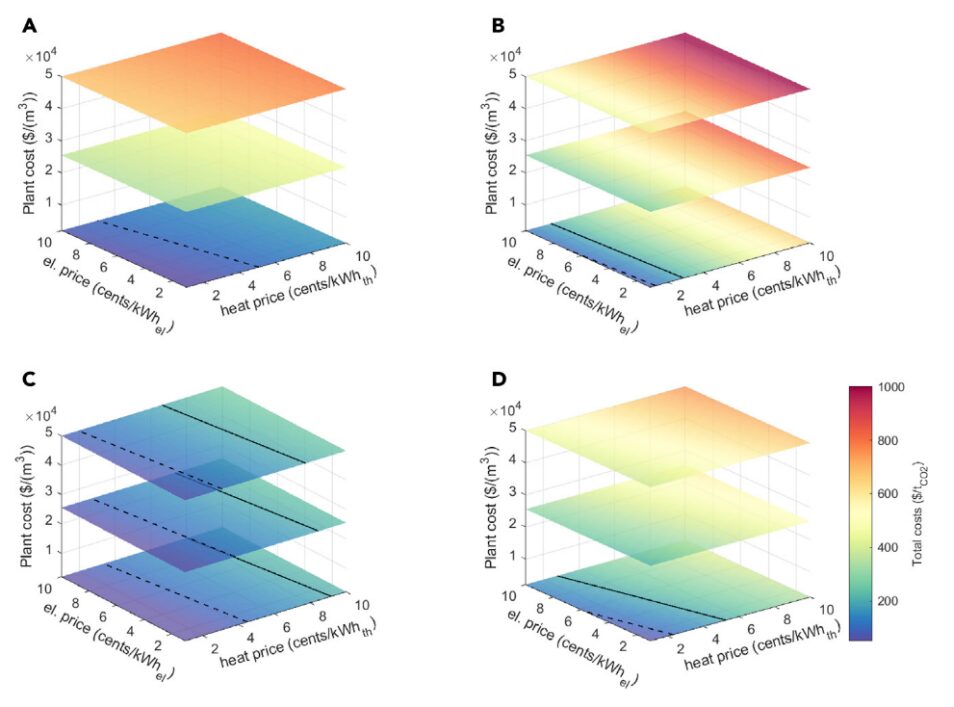
Figure 10: Total Costs of Three Different Process Systems as Functions of Electricity Price, Thermal Price, and Plant Costs. (A – D) (A) KOH using Point B on the Pareto graph (maximum productivity), (B) Amine washing using Point A on the graph (minimum exergy consumption), and (C and D) Solid adsorption scenarios E-A (C: High kinetics k = 0.1 s-1 using Point B on the Pareto graph and D: Low kinetics k = 0.0001 s-1 using a midpoint on the Pareto graph with exergy consumption similar to the KOH process). Assuming the plant operates at full capacity, with a 20-year project life and a 10% discount rate. The total cost values shown by the dashed line are $100/tCO2, and the continuous line shows the values at $200/tCO2.
From Figure 10, the following conclusions can also be drawn:
For the amine washing process (and similar liquid washing processes using amines as solvents), improvements must focus on reducing thermal energy consumption. New solvents need to combine high CO2 loading, rapid reaction, limited toxicity, and simple manufacturing. For the alkaline washing process, keeping contactor costs low is key. For solid adsorption processes, cost-optimized designs require a thorough characterization of the adsorbent from the perspective of multicomponent balance and transfer. Once the properties of the adsorbent are known, the focus of improvements can be on reducing contactor costs, such as through additive manufacturing or designing a convenient heat supply energy system, such as through integrated heat recovery options.
Productivity: The productivity range for both liquid-solvent washing processes is low, at [0.18-0.45 kgCO2m-3 h-1] for alkaline washing and [0.75-1.08 kgCO2m-3h-1] for amine washing. For solid adsorbent processes, this range is much broader, at [3.8-10.6 kgCO2m-3h-1], but there is more data uncertainty involved.
Energy Consumption: The amine washing process has the highest energy consumption, while solid adsorption processes have relatively low energy consumption and lower temperatures.
Scalability: Alkaline washing requires a complex process, making it more suitable for large plants. For amine washing, the regeneration process is relatively complex and requires specialized equipment (heat exchangers, steam strippers, etc.), making it unsuitable for small-scale applications. Solid adsorption processes can be easily scaled up or down, but the large number of pipelines and valves required for large-scale operation will make process design and control challenging.
Economic Performance: The costs of all three processes are below $200/ton CO2.
Scientific Challenges: The liquid-solvent washing process is well understood. For solid adsorption processes, there is a lack of experimental data related to DAC applications, and the potential adsorption mechanisms are not fully understood.
Technical Challenges: More efficient designs for different reactors in the regeneration segment, as well as the electrification of calcination furnaces, could further improve the alkaline washing process. For amine washing, the adsorber needs to be redesigned to handle more advanced amines (volatile, toxic, etc.). Challenging areas for solid adsorption processes include the development of efficient heat exchange within contactors, thermal integration (heating/cooling), and advanced designs for contactors.
Francesco Sabatino, Alexa Grimm, Fausto Gallucci, Martin van Sint Annaland, Gert Jan Kramer, and Matteo Gazzani, A comparative energy and costs assessment and optimization for direct air capture technologies, 2021, https://doi.org/10.1016/j.joule.2021.05.023
Figure 8: Pareto Curves for Solid Adsorption Optimization Using Various CO2 and H2O Isotherm Combinations
Figure 9: Detailed Energy and Exergy Demands for Different Solid Adsorption Processes. (A) Breakdown of Exergy Demand. (B) Breakdown of Energy Demand. Thermal energy demand is represented in blue, with different shades representing the reaction heat of CO2 and H2O (at equilibrium T=373 K, p = 0.1 bar), as well as the sensible heat of the adsorbent, CO2, and H2O. In both figures, X-X: A represents the minimum exergy/energy on the Pareto, and X-X: B represents maximum productivity.
Figure 10: Total Costs of Three Different Process Systems as Functions of Electricity Price, Thermal Price, and Plant Costs. (A – D) (A) KOH using Point B on the Pareto graph (maximum productivity), (B) Amine washing using Point A on the graph (minimum exergy consumption), and (C and D) Solid adsorption scenarios E-A (C: High kinetics k = 0.1 s-1 using Point B on the Pareto graph and D: Low kinetics k = 0.0001 s-1 using a midpoint on the Pareto graph with exergy consumption similar to the KOH process). Assuming the plant operates at full capacity, with a 20-year project life and a 10% discount rate. The total cost values shown by the dashed line are $100/tCO2, and the continuous line shows the values at $200/tCO2.
From Figure 10, the following conclusions can also be drawn:
For the amine washing process (and similar liquid washing processes using amines as solvents), improvements must focus on reducing thermal energy consumption. New solvents need to combine high CO2 loading, rapid reaction, limited toxicity, and simple manufacturing. For the alkaline washing process, keeping contactor costs low is key. For solid adsorption processes, cost-optimized designs require a thorough characterization of the adsorbent from the perspective of multicomponent balance and transfer. Once the properties of the adsorbent are known, the focus of improvements can be on reducing contactor costs, such as through additive manufacturing or designing a convenient heat supply energy system, such as through integrated heat recovery options.
Productivity: The productivity range for both liquid-solvent washing processes is low, at [0.18-0.45 kgCO2m-3 h-1] for alkaline washing and [0.75-1.08 kgCO2m-3h-1] for amine washing. For solid adsorbent processes, this range is much broader, at [3.8-10.6 kgCO2m-3h-1], but there is more data uncertainty involved.
Energy Consumption: The amine washing process has the highest energy consumption, while solid adsorption processes have relatively low energy consumption and lower temperatures.
Scalability: Alkaline washing requires a complex process, making it more suitable for large plants. For amine washing, the regeneration process is relatively complex and requires specialized equipment (heat exchangers, steam strippers, etc.), making it unsuitable for small-scale applications. Solid adsorption processes can be easily scaled up or down, but the large number of pipelines and valves required for large-scale operation will make process design and control challenging.
Economic Performance: The costs of all three processes are below $200/ton CO2.
Scientific Challenges: The liquid-solvent washing process is well understood. For solid adsorption processes, there is a lack of experimental data related to DAC applications, and the potential adsorption mechanisms are not fully understood.
Technical Challenges: More efficient designs for different reactors in the regeneration segment, as well as the electrification of calcination furnaces, could further improve the alkaline washing process. For amine washing, the adsorber needs to be redesigned to handle more advanced amines (volatile, toxic, etc.). Challenging areas for solid adsorption processes include the development of efficient heat exchange within contactors, thermal integration (heating/cooling), and advanced designs for contactors.
Francesco Sabatino, Alexa Grimm, Fausto Gallucci, Martin van Sint Annaland, Gert Jan Kramer, and Matteo Gazzani, A comparative energy and costs assessment and optimization for direct air capture technologies, 2021, https://doi.org/10.1016/j.joule.2021.05.023