Recently, a prospective study published by Professor Zhang Mingzhi, Professor Chen Qingjiang, and Professor Zhang Xudong from Zhengzhou University First Affiliated Hospital in Frontiers in Oncology (impact factor 6.244) evaluated the efficacy and safety of decitabine (DAC) combined with cisplatin, cytarabine, and dexamethasone (DHAP) modified regimen in patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) after second-line treatment failure. In 21 R/R DLBCL patients, the overall response rate (ORR) reached 50%, and the safety was good, making it a promising new option for treating R/R DLBCL patients after second-line treatment failure.
Methods
1. Inclusion Criteria for Patients:
All enrolled R/R DLBCL patients had received at least one salvage therapy after standard treatment. Patients aged 14 to 65 years, with ECOG scores ≤2, had relapsed or failed to achieve CR after R-CHOP or similar first-line treatment, failed second-line treatment, and had not received DHAP treatment previously. The enrolled patients met the hematological criteria for chemotherapy, with an expected survival time of over 3 months, and had at least one measurable lesion. Patients who had received prior radiotherapy, had primary/secondary central nervous system lymphoma, had any uncontrolled other diseases (such as uncontrolled diabetes, severe heart failure, severe infections), or had a history of other tumors were excluded.
2. Study Design and Treatment Regimen
DAC 10mg/d for a total of 5 days, followed by a modified DHAP regimen. For patients who responded to rituximab in first-line treatment, rituximab 375mg/m2 was given before DHAP. The modified DHAP regimen included: cisplatin 100mg/m2, intravenous injection on days 1-3; cytarabine 2g/m2, intravenous injection every 12 hours; dexamethasone 40mg/d, intravenous injection on days 1-4. The treatment consisted of 4 cycles (each cycle lasting 21 days). After the second and fourth cycles, CT scans or PET scans were used to detect residual tumors and response to treatment.
Results
1. Baseline Characteristics of Patients
Among 21 R/R DLBCL patients, 1 patient withdrew after 1 cycle of treatment, and 20 patients received at least two cycles of treatment for efficacy and safety evaluation. At the end of treatment, 5 patients were assessed using PET, while the others were assessed using CT. The median age of the 20 patients was 50.5 years (range 30-65 years). The male-to-female ratio was 0.538:1. 15 patients (75%) had stage III/IV disease. 10 patients (50%) had intermediate to high-risk IPI scores. 12 patients (60%) had elevated lactate dehydrogenase (LDH), and 17 patients (85%) had primary refractory disease. All patients had undergone adequate standard treatment and received at least 1 salvage therapy before enrollment, with 6 patients having received more than two salvage therapies prior to enrollment.
Table 1. Baseline Characteristics of Patients

2. Efficacy and Survival Rates
As of the data cut-off date (October 2020), the median number of treatment cycles was 3 (range 2-4), with an ORR of 50% and a complete response (CR) rate of 35%. 5 patients (25%) achieved stable disease (SD), resulting in a disease control rate (DCR) of 75%. Subgroup analysis showed that patients over 50 years had a higher CR rate compared to younger patients (P=0.005), and relapsed patients had a better CR rate than those with primary refractory disease (P=0.031). The median follow-up time was 7.5 months (range 1.5-28 months). The median progression-free survival (PFS) was 7 months, and the median overall survival (OS) was not reached. The one-year OS rate was 59.0%, and the two-year OS rate was 51.6%.
Table 2. Response Rates of All Patients
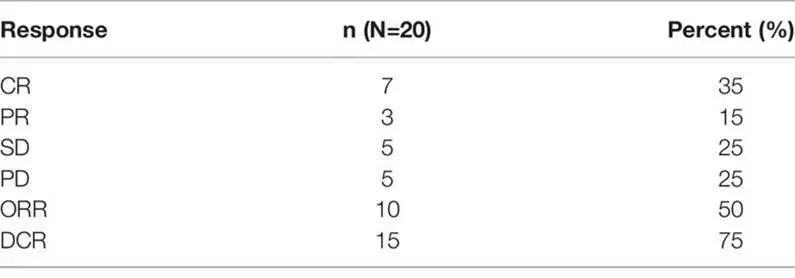
Table 3. Response Rates of Patients in Each Subgroup
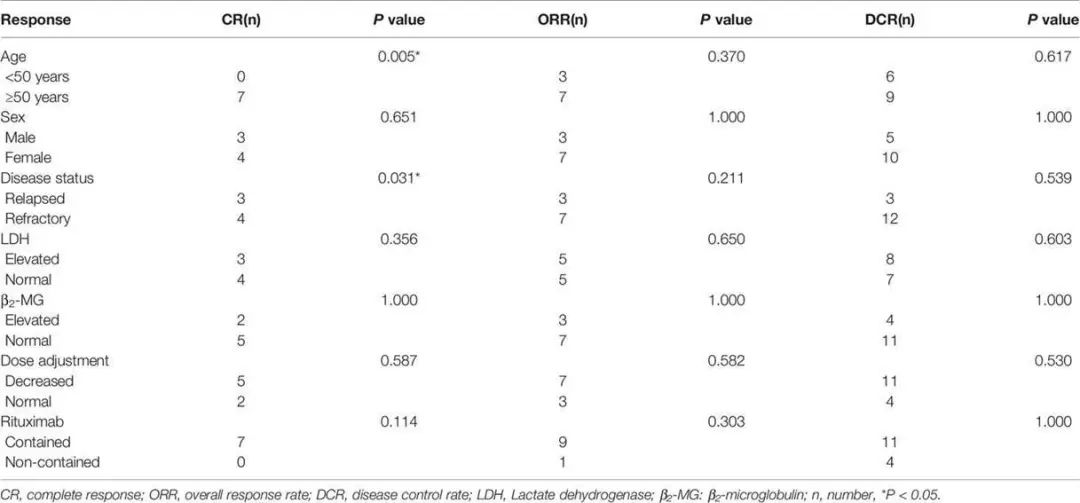
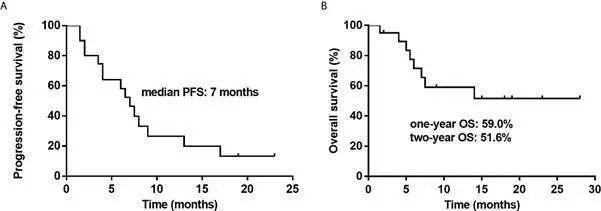
Figure 1. Kaplan-Meier Survival Curves for All Patients. A: PFS for All Patients. B: OS for All Patients.
3. Safety and Adverse Events
The main adverse events (AEs) observed were myelosuppression, gastrointestinal events, and infections due to neutropenia. The most common grade 3/4 hematological AEs were neutropenia (90%), anemia (50%), and thrombocytopenia (70%), most of which were alleviated after symptomatic treatment with rhG-CSF, rhTPO, and transfusions. Appropriate dose reductions of 20% were made for patients with grade 4 myelosuppression. Other major non-hematological AEs included grade 1/2 nausea/vomiting (40%) and infections due to neutropenia (50%). 4 patients (20%) experienced mild hyperbilirubinemia, and 5 patients (25%) had elevated transaminases (AST/ALT). 1 patient (5%) experienced thrombocytopenia and gastrointestinal bleeding during treatment, and 1 patient (5%) experienced mild otitis media. There were no significant nephrotoxic or treatment-related death events during treatment.
Table 4. Summary of Adverse Events
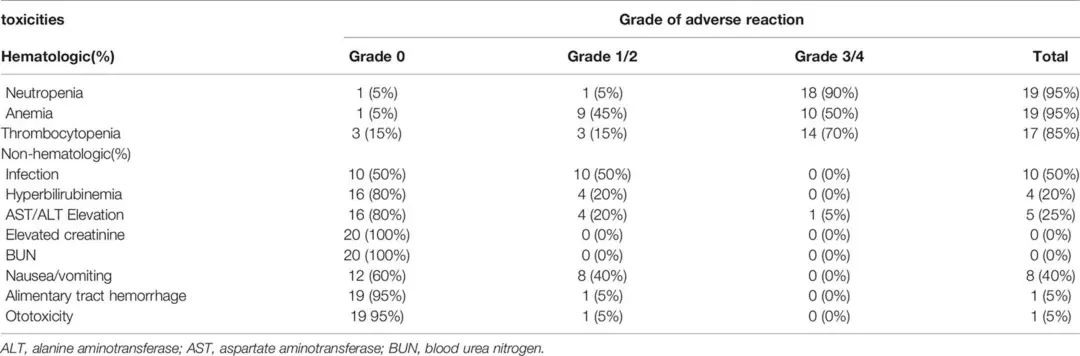
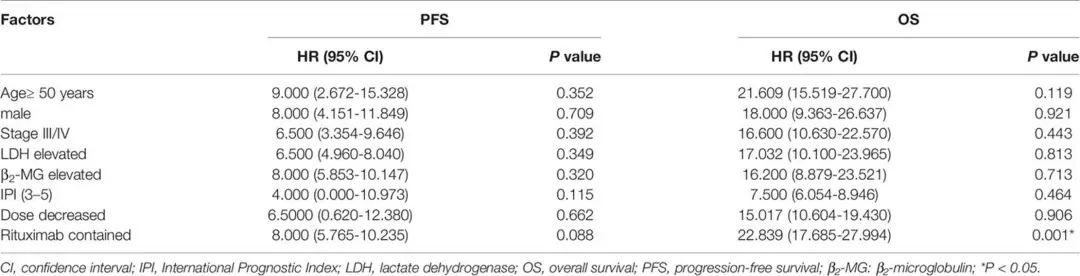
4. Possible Prognostic Factors
Univariate analysis was conducted to evaluate the correlation between clinical characteristics and survival prognosis. The analysis showed that patients using rituximab had better OS than those who did not (P=0.001). Age, disease stage, LDH level, IPI score, and dose adjustment had no significant correlation with PFS or OS.
Table 5. Univariate Analysis of Prognostic Factors for PFS and OS
Researcher’s Perspective
1. R/R DLBCL is currently a key challenge in DLBCL treatment, especially for patients with failed salvage therapy, where treatment options are limited, and most patients are in poor physical condition, making them difficult to tolerate high-dose chemotherapy or hematopoietic stem cell transplantation, ultimately leading to poor prognosis. This study adopted a low-dose DAC combined with a modified DHAP regimen, with the vast majority of patients able to tolerate up to two cycles for efficacy evaluation, without significantly increasing chemotherapy adverse reactions, indicating good safety. The overall response rate (ORR) was 50%, and the complete response (CR) rate reached 35%, with a two-year OS rate of 51.6%, better than historical studies of similar types. This indicates that the combination of decitabine and cytarabine-containing DHAP regimen has a more promising synergistic effect and may achieve better depth of response.
2. Currently, there is increasing evidence regarding the enhancing effect of low-dose DAC on chemotherapy, immunotherapy, and other epigenetic drugs, including clinical studies in solid tumors and hematological tumors showing that low-dose DAC has good safety, thus can be combined with many treatment modalities. We believe that future research on DAC in lymphoma will become more widespread, with deeper insights into the mechanisms involved, and research on other epigenetic drugs like HDACi will also have a positive impact, providing more optimized options for cancer treatment and potentially bringing benefits to more patients.
Hu J, Wang X, Chen F, Ding M, Dong M, Yang W, Yin M, Wu J, Zhang L, Fu X, Sun Z, Li L, Wang X, Li X, Guo S, Zhang D, Lu X, Leng Q, Zhang M, Zhu L, Zhang X and Chen Q. Combination of Decitabine and a Modified Regimen of Cisplatin, Cytarabine and Dexamethasone: A Potential Salvage Regimen for Relapsed or Refractory Diffuse Large B-Cell Lymphoma After Second-Line Treatment Failure. Front. Oncol. 18 June 2021. https://doi.org/10.3389/fonc.2021.687374
