With the widespread use of electronic products and the rapid popularization of new energy vehicles, it can be said that no era has been more concerned with battery energy density than ours today.
The well-known ternary lithium battery can achieve an energy density of 300 watt-hours per kilogram, while a less mature technology, the lithium-sulfur battery, can easily achieve an energy density of 600 watt-hours per kilogram, with a theoretical energy density that is astonishingly high!
The “Lithium-Sulfur Battery White Paper” published by the International Battery Materials Association states that the theoretical energy density of lithium-sulfur batteries is 2600 watt-hours per kilogram!
Such an enticing energy density is bound to attract researchers from various countries. On February 29 of this year, the National Natural Science Foundation of China released the “Top Ten Scientific Advances in China for 2023,” and a study on lithium-sulfur batteries successfully made the list. Today, let’s take a closer look at this type of battery.
Family Elements
Among the various batteries under research, the one with the highest energy density is not the lithium-sulfur battery, but the lithium-air battery, which has a theoretical energy density exceeding 3500 watt-hours per kilogram, significantly higher than that of lithium-sulfur batteries.
The principle is to use lithium as the negative electrode material and oxygen from the air as the positive electrode material. During discharge, oxygen reacts with lithium ions to form lithium peroxide under the action of a catalyst; during charging, lithium oxide decomposes to generate oxygen and lithium ions.
Undoubtedly, this type of battery currently faces numerous technical challenges, such as:
▶ The lithium oxide generated during discharge can accumulate, thereby hindering the battery’s charge and discharge efficiency.
▶ Moisture and impurities in the air can corrode the battery, shortening its lifespan.
Therefore, current laboratory research on “lithium-air batteries” is often conducted in a “pure oxygen” environment. Perhaps success will come in the future, but for now, lithium-air batteries are not a mature technology we can achieve.
Since using “oxygen” as the positive electrode for lithium batteries is too advanced, is there a more realistic material? Of course, there is.
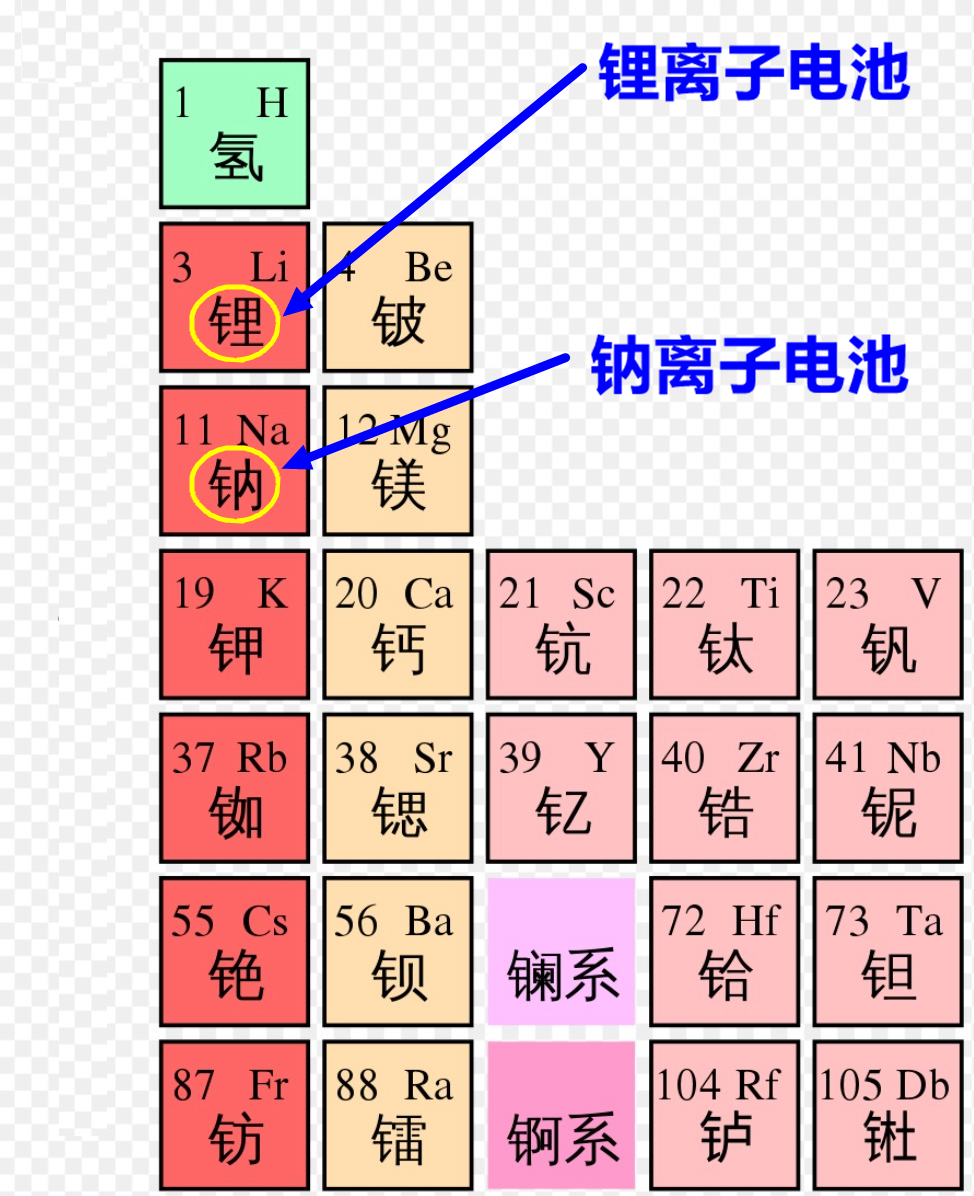
Periodic table of elements, image from Wikipedia.
In the periodic table, lithium and sodium belong to the same group of elements, possessing similar chemical properties. Therefore, with the widespread use of lithium-ion batteries today, sodium-ion batteries are also gradually moving towards commercialization, with promising prospects.
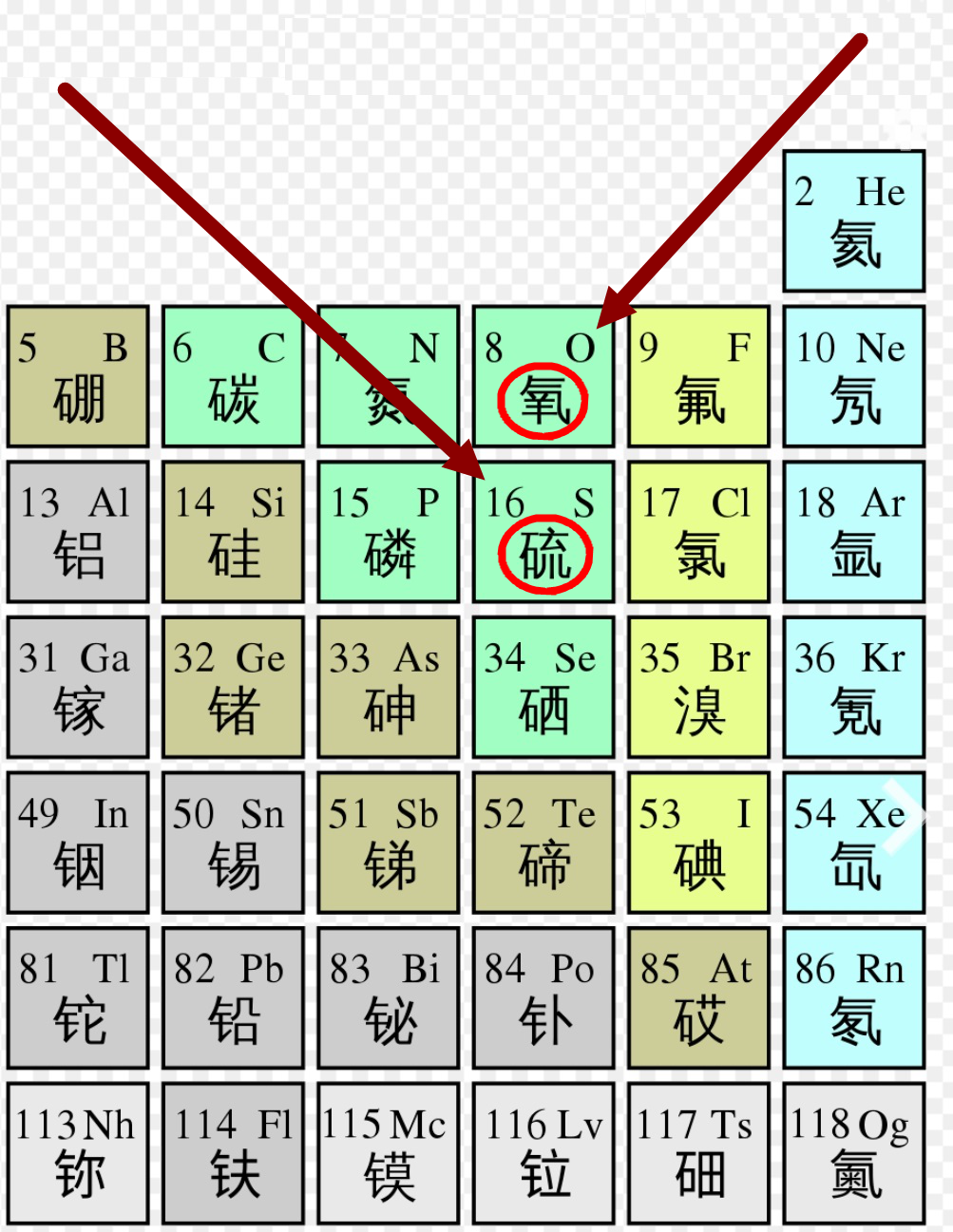
Periodic table of elements, image from Wikipedia.
In the periodic table, oxygen and sulfur also belong to the same group of elements, sharing similar chemical properties. Since “oxygen” can serve as the positive electrode in lithium-air batteries, the group element sulfur can also serve as the positive electrode in batteries—this is the lithium-sulfur battery.
History of Lithium-Sulfur Batteries
Research on lithium-sulfur batteries began in the 1960s.
In 1967, Herbert and Ulam first proposed that sulfur could be used as the positive electrode material for lithium batteries. It is important to note that the “lithium-sulfur battery” proposed at that time was still a primary battery, meaning it was a one-time-use battery.
In the 1980s, Plichta and others studied the charge and discharge mechanisms of lithium-sulfur batteries.
Since the 1990s, significant progress has been made in lithium-sulfur battery research, with energy density continuously increasing. However, the safety and economic viability of lithium-sulfur batteries remain poor.
After 2014, lithium-sulfur batteries began to enter a phase of small-scale experimental applications.
Applications in Large Solar Drones

The image shows the Zephyr series of large solar drones designed and manufactured by Airbus, image from Wikipedia.
In 2014, the large solar drone Zephyr 7, also known as “Zhongfeng 7,” used lithium-sulfur batteries to fly continuously for 11 days. The lithium-sulfur batteries provided by Sion Power at that time had an energy density of up to 350 watt-hours per kilogram.
While 350 watt-hours per kilogram may seem average, it is important to note that this was in 2014, ten years ago. At that time, new energy vehicles were just starting out, and the lithium-ion batteries in use had energy densities that now seem pitiful.
Although the Zhongfeng 7 used lithium-sulfur batteries, the newer “Zephyr S,” also known as “Zhongfeng 8,” achieved 64 days of continuous high-altitude flight in 2022, but did not use lithium-sulfur batteries. This indirectly indicates that lithium-sulfur batteries are still in the phase of small-scale experimental applications.
In 2020, the high-altitude solar drone “EAV-3,” developed by the Korea Aerospace Research Institute and equipped with lithium-sulfur batteries, successfully conducted stratospheric flight tests.

EAV-3 solar drone, image from Wikipedia.
In the 2020 flight test, the EAV-3 reached a maximum altitude of 22 kilometers. During the total flight time of 13 hours, the drone stably flew for 7 hours at altitudes between 12 kilometers and 22 kilometers in the stratosphere.
Advantages of Lithium-Sulfur Batteries
From the above, we can see that lithium-sulfur batteries are currently in a phase of small-scale experimental applications. Compared to traditional lithium-ion batteries, they have the following two core advantages:
1. The theoretical energy density of lithium-sulfur batteries far exceeds that of traditional lithium-ion batteries.
Ten years ago, lithium-sulfur batteries had already achieved 350 watt-hours per kilogram, while current traditional lithium-ion batteries have not surpassed this energy density.
Energy density, also known as “mass energy density,” refers to the energy possessed per unit mass. For example:
For two battery packs of equal mass, if battery pack A has an energy density of 200 watt-hours per kilogram and battery pack B has an energy density of 400 watt-hours per kilogram, this means that under the same usage conditions, the endurance time of battery pack A will be twice that of battery pack B.
Large solar drones flying above 20,000 meters operate in extremely thin air, making their weight crucial. Therefore, they initially attempted to use lithium-sulfur batteries with the core goal of making the battery pack as light as possible while maximizing energy storage capacity.

Yellow sulfur burns to form a blood-red liquid and emits blue flames. Image from Wikipedia.
2. The “sulfur” material in lithium-sulfur batteries is extremely inexpensive and has abundant global reserves.
If the technology for lithium-sulfur batteries matures and achieves large-scale use in the future, it is unlikely to be troubled by issues of sulfur scarcity and price.

A man carrying sulfur blocks from a volcano in Indonesia, image from Wikipedia.
Current Challenges of Lithium-Sulfur Batteries
The volume difference between elemental sulfur and lithium sulfide is significant, leading to about 80% volume expansion during the reduction reaction from elemental sulfur to “lithium sulfide.”
▶ Volume Expansion
In other words, lithium-sulfur batteries tend to be larger. This is not a major issue for large solar drones, as their size can accommodate the battery’s volume expansion.
However, for commonly used devices like smartphones or cars, this can be problematic, especially for smartphones, which have strict size limitations for batteries.
▶ Shuttle Effect
Volume expansion is not the biggest challenge; the most significant technical difficulty for lithium-sulfur batteries is the “lithium polysulfide shuttle effect.”
During the charge and discharge process of lithium-sulfur batteries, the intermediate product lithium polysulfide dissolves in the electrolyte and migrates to the negative electrode, where it reacts with lithium metal to form new lithium sulfide. This process, known as the “lithium polysulfide shuttle effect,” leads to rapid capacity decay and shortened cycle life.
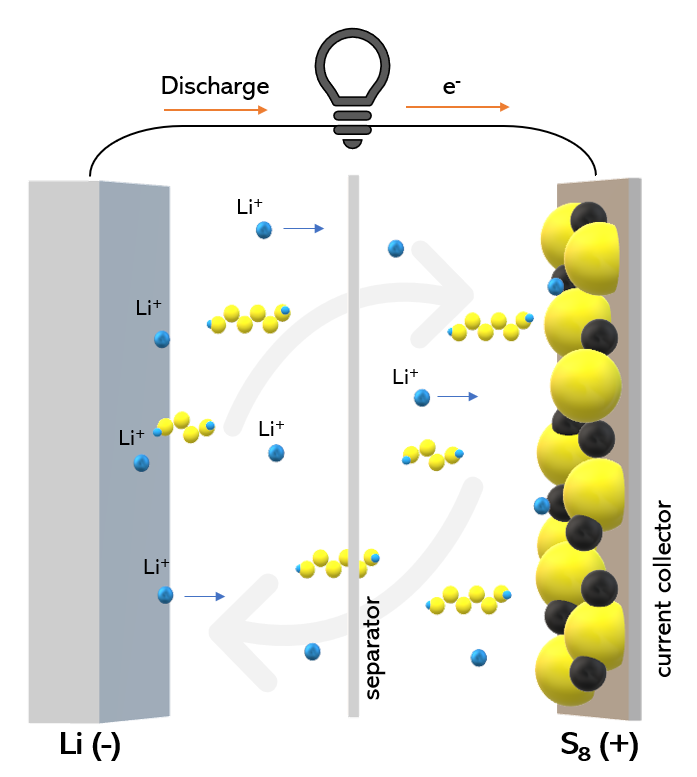
Working principle of lithium-sulfur batteries and the “shuttle” effect, image from Wikipedia.
Latest Developments
To address current technical challenges, researchers need to gain a clearer understanding of the chemical reactions occurring within lithium-sulfur batteries in order to solve problems effectively.
However, due to the low spatiotemporal resolution of traditional in-situ microscopic research techniques and the instability of the lithium-sulfur system, achieving this is quite difficult.
In the “Top Ten Scientific Advances in China for 2023,” researchers Liao Honggang and Sun Shigang from Xiamen University, along with Chen Jianfeng from Beijing University of Chemical Technology, developed a high-resolution electrochemical in-situ transmission electron microscopy technique that enables atomic-scale dynamic real-time observation and study of interfacial reactions in lithium-sulfur batteries.
Moreover, for nearly a century, “electrochemical interfacial reactions” have typically been thought to occur only through “inner-sphere reactions” and “outer-sphere reactions” as single-molecule pathways.
This time, the research by Chinese scientists revealed the existence of a third pathway, which is the “charge storage aggregation reaction.”
Undoubtedly, this new discovery will provide guidance for the future design of lithium-sulfur batteries.
References:
[1]https://sionpower.com/2014/sion-powers-lithium-sulfur-batteries-power-high-altitude-pseudo-satellite-flight/
Author: Han Mu Diao Meng, Science Writer, Winner of the “National Excellent Science Popularization Works Award” from the Ministry of Science and Technology
Reviewed by: Zhang Haijun, Professor at the School of Safety Science and Engineering, Civil Aviation University of China, Deputy Secretary-General of Tianjin Emergency Management Society, Postdoctoral Fellow at the Department of Chemistry, University of Puerto Rico
Produced by: Science Popularization China
Supervised by: China Science and Technology Publishing House Co., Ltd., Zhongke Shuchuang (Beijing) Digital Media Co., Ltd.
Reformatted and edited by Science Popularization China
Content from: Science Popularization China
Content resources provided by the project unit