*The professional content discussed in this article is intended for reference by medical professionals only.

Glucokinase (GK) can sense glucose concentration and regulate the timing and level of insulin secretion from pancreatic β-cells, playing a core role as a “component” in the intelligent regulation of blood glucose homeostasis in the human body. Regulating glucokinase (GK) in human pancreatic cells and other tissues can correct metabolic defects in type 2 diabetes mellitus (T2DM). Based on this theory, the clinical development of glucokinase activators (GKA) has been proposed and promoted, leading to Professor Franz Matschinsky of the University of Pennsylvania receiving the “Nobel Prize” in endocrinology—the Rolf Luft Award.Among them, the globally first innovative drug under research by Hualing Pharmaceutical, Dorzagliatin, is currently the only glucokinase activator (GKA) that has entered and completed phase III clinical trials. Professor Franz Matschinsky, along with prominent figures such as Professors Zhu Dalong and Yang Wenying from China, participated in its clinical research. Why does Dorzagliatin stand out among the GKA that once sparked a global research boom? What are its clinical efficacy and safety? At the 24th National Academic Conference of the Diabetes Society of the Chinese Medical Association in 2020, Dr. Chen Li, Chief Scientific Officer of Hualing Pharmaceutical, shared insights on the treatment needs for T2DM, the current status of GKA research, and the kinetic differences among various glucokinase activators (GKA).
Repairing Glucose Sensors,
Helping to Treat T2DM from the Source
Glucose is the core energy resource for human survival and progress. During the storage, synthesis, and utilization of glucose, hexokinases I-IV (HK I-IV) play different roles. Among them, hexokinase IV, or glucokinase (GK), is crucial for maintaining blood glucose homeostasis in the human body. Glucokinase (GK) is mainly distributed in the pancreas, liver, and intestines, and its activation is determined by glucose concentration, which in turn regulates the secretion of glucose-controlling hormones, namely insulin and glucagon, as well as glycogen synthesis, keeping blood glucose levels within the target range of 4-6.5 mmol/L1, consistent with the target range for time in range (TIR) of blood glucose. In other words, glucose acts as the first messenger in glucose metabolism, and glucokinase (GK) functions as a glucose sensor.For example, when blood glucose is too high, the activity of glucokinase (GK) in pancreatic β-cells increases, regulating the timely and adequate secretion of insulin; the activity of glucokinase (GK) in liver cells increases, promoting glycogen synthesis; simultaneously, the activity of glucokinase (GK) in α-cells enhances, inhibiting glucagon release. Conversely, when blood glucose is too low, the activity of glucokinase (GK) in α-cells decreases, promoting glucagon release, which raises blood glucose to physiological target levels2. This is similar to how sensors in smart air conditioners perceive and transmit temperature, allowing the air conditioner to maintain the room within the set temperature range.Research shows that T2DM patients have significantly reduced expression of glucokinase (GK) in the pancreas and liver, leading to decreased sensitivity of the homeostatic regulation system to blood glucose changes, closely related to hepatic insulin resistance and the onset of diabetes3,4,5. Therefore, impaired glucokinase (GK) function is a core cause of the occurrence and development of T2DM.In the absence of existing diabetes treatment drugs and therapies that fully restore β-cell function, glucokinase activators (GKA) have the potential to repair the glucose sensor function of glucokinase (GK), restoring the intelligent regulation of blood glucose in T2DM patients, thereby treating diabetes from its source.
Different Glucokinase Activators (GKA),
Dorzagliatin (Dorzagliatin)
Stands Out
After Professor Franz Matschinsky proposed the theory that “glucokinase (GK) has a core function in blood glucose homeostasis,” the development of glucokinase activators (GKA) received a significant boost, leading to a global research boom after 2003.In the 20 years since the birth of GKA, multinational companies such as Roche, Pfizer, AstraZeneca, Merck, and Hualing have invested in research and development, resulting in four generations of glucokinase activators (GKA):
-
First generation (1998-2002): Roche’s Ro 0281675, which entered phase I clinical trials;
-
Second generation (2000-2020): Piragliatin, pf04937319, AZD1656, and MK0941, which entered phase II clinical trials;
-
Third generation (2004-2020): RO4597014, which entered phase II clinical trials;
-
Fourth generation (2012-2020): Dorzagliatin, which is currently the only glucokinase activator (GKA) to have entered and completed phase III clinical trials.
Based on chemical structure, glucokinase activators (GKA) can be divided into benzamide (e.g., AZD1656 and MK0941), phenethylamide (e.g., Roche’s first and second generation GKA), and amino acid amide (Dorzagliatin). The different chemical structures of glucokinase activators (GKA) have a significant impact on their pharmacological and biological activities.Roche’s second-generation GKA completed phase II clinical trials and showed good hypoglycemic efficacy, but the drug’s human metabolites exceeded the concentration of the original drug, leading to accumulation in the body after redox reactions with the metabolites, posing safety risks, and ultimately halting development6.Pfizer’s GKA (pf04937319) is still under investigation; reported in 20157, this drug, based on metformin, combined with sulfonylureas and sitagliptin, showed a reduction in glycosylated hemoglobin (HbA1c) comparable to sitagliptin but inferior to glimepiride. The efficacy of pf04937319 in lowering fasting blood glucose was less than that of sitagliptin and glimepiride, with a low risk of hypoglycemia comparable to sitagliptin and much lower than glimepiride, indicating limited hypoglycemic efficacy but low hypoglycemia risk.AstraZeneca’s AZD1656 phase II clinical study found that in untreated T2DM patients in Japan, AZD1656 could lower fasting blood glucose but rapidly lost efficacy after one month, while also increasing fasting insulin secretion7. Consequently, clinical research on this drug was also terminated.Merck’s MK0941 phase II clinical study showed that in T2DM patients with poor insulin control, treatment with MK0941 did not sustain hypoglycemic efficacy, with a hypoglycemia incidence rate as high as 30%. This was related to MK0941 significantly altering the nH (Hill coefficient) of glucokinase (GK) and interfering with glucose-dependent kinase activation, as well as MK0941’s strong activation of glucokinase (GK) at low blood glucose (2.5 mmol/L) and weak activation at high blood glucose (10 mmol/L)8, leading to the termination of MK0941’s development.In contrast to previous GKAs, Dorzagliatin stands out by focusing on enhancing pancreatic sensitivity to glucose and hypoglycemic effects during clinical development. In phase I-III clinical studies, it has demonstrated good hypoglycemic efficacy and safety, with a dose-dependent hypoglycemic effect. Research indicates that Dorzagliatin enhances glucokinase (GK) expression in the liver of diabetic rats and improves early-phase insulin secretion in T2DM patients9,10. During the study, whether as monotherapy or in combination with metformin, it significantly reduced HbA1c and postprandial blood glucose, significantly improving HOMA-2β, with stable and sustained HbA1c reduction over 52 weeks of monotherapy (Figure 1)11.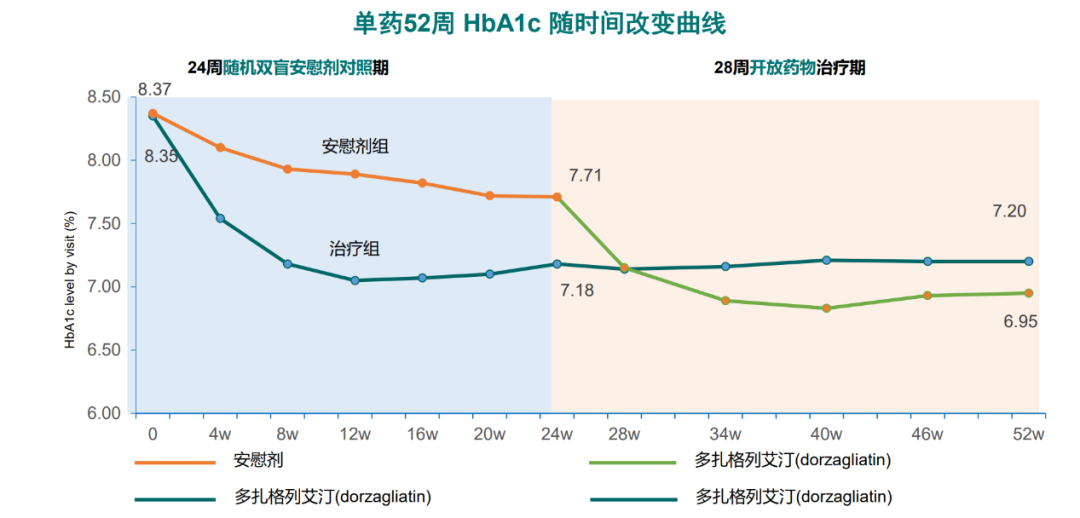 Figure 1 The long-term hypoglycemic effect of Dorzagliatin (Dorzagliatin) monotherapy remains stable
Figure 1 The long-term hypoglycemic effect of Dorzagliatin (Dorzagliatin) monotherapy remains stable
Dual Activation, Excellent Kinetics:
Uncovering the “Core Competence” of Dorzagliatin (Dorzagliatin)
What are the “core competencies” of Dorzagliatin (Dorzagliatin) that make it stand out among glucokinase activators (GKA)?
An ideal glucokinase activator (GKA) can alter the kinetic parameters of glucokinase (GK), increase the maximum working efficiency (Vmax), and enhance conversion efficiency without affecting the binding ability of glucose to glucokinase (GK), thereby not altering nH and maintaining the glucose sensor function of glucokinase (GK). Among many glucokinase activators (GKA), Pfizer’s pf04937319 and AstraZeneca’s AZD1656 cannot enhance the catalytic efficiency of glucokinase (GK), while Merck’s MK0941 significantly alters nH. In contrast, Dorzagliatin, which has dual effects on the pancreas and liver, fully meets the conditions required for an ideal glucokinase activator (GKA), which is the reason for its successful clinical research12.
Conclusion
Glucokinase (GK) is the core component for maintaining blood glucose homeostasis in the human body, and its dysfunction is a core cause of the occurrence and development of T2DM. The glucokinase activator (GKA) Dorzagliatin (Dorzagliatin) acts simultaneously on the pancreas and liver, exhibiting excellent kinetic characteristics, thus standing out among many glucokinase activators (GKA) and possessing the potential to repair the sensor function of glucokinase (GK), enhancing the intelligent regulation of blood glucose in T2DM patients and fundamentally treating diabetes.nH is one of the key parameters of glucokinase (GK) as a blood glucose sensor, affecting the cooperativity of glucose regulation on glucokinase (GK) function, with a normal value of 1.7. If a glucokinase activator (GKA) significantly alters nH, it is equivalent to setting the blood glucose target range far from the normal range of 4-6.5 mmol/L. When nH changes from 1.7 to close to 1 (as with MK0941), the human blood glucose range would be set in the hypoglycemic range of 2-4 mmol/L, thereby increasing the risk of hypoglycemia.Vmax represents the maximum working efficiency of glucokinase (GK). An ideal glucokinase activator (GKA), such as Dorzagliatin (Dorzagliatin), can enhance the Vmax of glucokinase (GK), gradually repairing its sensing function and increasing the expression of glucokinase (GK), restoring the function and quantity of damaged glucokinase (GK) in T2DM patients.Expert Profile
Dr. Chen Li
Chief Scientific Officer of Hualing Pharmaceutical
Chairman, CEO, Founder, and Chief Scientific Officer of Hualing Pharmaceutical Technology (Shanghai) Co., Ltd.
Former part-time professor and doctoral supervisor at the School of Life Sciences, Tongji University, and part-time researcher at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
Former Chief Scientific Officer at Roche R&D China, with over 20 years of experience in new drug research and development innovation and management.
Dr. Chen Li founded Hualing Pharmaceutical in 2011, which went public on the Hong Kong Stock Exchange in 2018, with a market value of $1 billion.
Dr. Chen Li obtained his Ph.D. from Iowa State University in 1992 and joined Roche’s U.S. R&D center the same year.
He has held positions as project manager, director of high-throughput technology, and Chief Scientific Officer at Roche’s China R&D center.
Former chairman of the China-U.S. Pharmaceutical Association, playing an important role in promoting exchanges and cooperation in the field of new drug research and development between China and the U.S., and making significant contributions to major institutional breakthroughs such as the national MAH.
Dr. Chen Li is the inventor of 40 authorized invention patents and 84 invention patent applications, and has published over 60 scientific papers in renowned international journals, including Lancet Diabetes and Endocrinology, Diabetes, Obesity and Metabolism, J Diabetes Res., Clinical Pharmacokinetics, PNAS, J Med Chem, J Am Chem Soc, J. Org Chem.
References:
- Irwin DM, Tan H. Molecular evolution of the vertebrate hexokinase gene family: Identification of a conserved fifth vertebrate hexokinase gene[J]. Comp Biochem Physiol Part D Genomics Proteomics, 2008, 3(1):96-107.
- Matschinsky FM, Wilson DF. The central role of glucokinase in glucose homeostasis: a perspective 50 years after demonstrating the presence of the enzyme in islets of Langerhans[J]. Front Physiol, 2019, 10:148-163.
- Li C, Liu C, Nissim I, et al. Regulation of glucagon secretion in normal and diabetic human islets by γ-hydroxybutyrate and glycine[J]. J Biol Chem, 2013, 288(6):3938-51.
- Haeusler RA, Camastra S, Astiarraga B, et al. Decreased expression of hepatic glucokinase in type 2 diabetes[J]. Mol Metab. 2014, 4(3):222-6.
- Jiang MH, Fei J, Lan MS, et al. Hypermethylation of hepatic Gck promoter in ageing rats contributes to diabetogenic potential[J]. Diabetologia, 2008, 51(8):1525-33.
- Sarabu R, Bizzarro FT, Corbett WL, et al. Discovery of piragliatin–first glucokinase activator studied in type 2 diabetic patients[J]. J Med Chem, 2012, 55(16):7021-36.
- N. B. Amin, et al. Diabetes, Obesity and Metabolism 2015
- Meininger GE, Scott R, Alba M, et al. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes[J]. Diabetes Care, 2011, 34(12):2560-6.
- Wang P, Liu H, Chen L, et al. Effects of a Novel Glucokinase Activator, HMS5552, on Glucose Metabolism in a Rat Model of Type 2 Diabetes Mellitus[J]. J Diabetes Res, 2017, 2017:5812607.
- Bergstrom RW, Wahl PW, Leonetti DL, Fujimoto WY. Association of fasting glucose levels with a delayed secretion of insulin after oral glucose in subjects with glucose intolerance[j]. J Clin Endocrinol Metab, 1990, 71(6):1447-53.
- Zhu D, Gan S, Liu Y, et al. Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: a dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study[J]. Lancet Diabetes Endocrinol, 2018, 6(8):627-636.
- Yongqiang Shan, Xiaowei Jin, Xiaobing Lv, Li Chen. Dorzagliatin differentiates from early generation of glucokinase activators: an enzyme kinetics study. Poster: 1151-P, American Diabetes Association (ADA) 79th Scientific Sessions, June 7-11, 2019, San Francisco, USA
Previous Highlights



