
In recent years, touch screens have become increasingly common in electronic devices, appearing not only in smartphones and tablets but also in navigation systems, ATMs, and other equipment. Unlike traditional input methods such as keyboards and mice, touch screens allow users to perform various operations simply by tapping or swiping with their fingers, making electronic devices more convenient and compact. However, many people may not realize that the technology behind touch screens relies on an important material: indium tin oxide (ITO).
Why is indium tin oxide crucial for touch screens? To answer this question, we first need to understand a bit about how touch screens work. There are many ways to implement touch screens, but the two main types are resistive and capacitive, both of which rely on changes in electrical signals triggered by the contact of a finger or other object with the screen.
Let’s first look at resistive touch screens. As shown in Figure 1, the upper layer of the touch screen is a transparent plastic, while the lower layer is glass (which can also be plastic). Each surface of the upper plastic material and the lower glass has a thin layer of conductive material, separated by numerous insulating points; therefore, when there is no external force, these two conductive layers do not come into contact. Once we gently tap the upper plastic with our finger, it deforms under pressure, causing the two conductive materials to make contact, resulting in a change in the circuit’s current. This change in current is then converted into a digital signal through a series of carefully designed circuits. Thus, by tapping with our fingers, we can perform operations on electronic devices.

Figure 1 Basic Structure of Resistive and Capacitive Touch Screens
The structure of capacitive touch screens is similar to that of resistive touch screens, but instead of measuring changes in resistance and current, they measure changes in capacitance. Everyone knows that if two parallel conductors are separated by an insulator, the simplest capacitor is formed, capable of storing a certain amount of charge. When we separate two sheets of glass or plastic coated with conductive material using an insulating material and allow the two conductive layers to be relative, we create such a capacitor. Since the human body is also a conductor, touching one of the glass sheets with a finger creates a new capacitor between the finger and the conductive material on the glass. This change in the touch screen’s charge can then be converted into the corresponding digital signal, thus completing the operation.
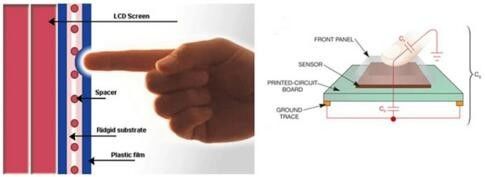
Figure 2 Basic Working Principles of Resistive and Capacitive Touch Screens.
Of course, whether it is a resistive or capacitive touch screen, what we are introducing here is only the most basic working principle; in reality, the structure is much more complex. Here lies a critical issue: both glass and most plastics are insulators. To ensure the normal operation of touch screens, we must cover their surfaces with a layer of conductive material, as mentioned earlier. Some may suggest that sticking a layer of aluminum foil or attaching a copper plate to the glass or plastic would suffice. While this indeed solves the conductivity issue, it renders the entire touch screen opaque, and even if it operates normally, it has no practical value. Therefore, to ensure the normal operation of touch screens, we must find a material that guarantees good conductivity while also being sufficiently transparent.
This special requirement of touch screens has posed a significant challenge for scientists. Fortunately, through continuous exploration, scientists have discovered that some metal oxides can simultaneously meet the conditions of conductivity and transparency, with indium tin oxide being one of the best. From its name, indium tin oxide is a compound composed of indium, tin, and oxygen, but it is actually a mixture of indium oxide and tin oxide in a specific ratio, with tin oxide generally accounting for 10% of the total weight. The indium tin oxide obtained from manufacturers is usually a white, gray, or yellow powder; however, when applied to the surface of glass or plastic, it forms a transparent film (Figure 3). When the film’s thickness does not exceed a few hundred nanometers (one nanometer is one-billionth of a meter), it can ensure that 80% or more of visible light passes through, thus having minimal impact on the light transmission of the glass or plastic. Meanwhile, this layer of indium tin oxide film has strong conductivity, with resistivity as low as one ten-thousandth of an ohm meter. The perfect combination of transparency and conductivity makes indium tin oxide the ideal choice for producing touch screens.

Figure 3 Left: Solid Indium Tin Oxide. Right: Glass Coated with Indium Tin Oxide.
Besides touch screens, many other electronic products also rely on indium tin oxide. For example, liquid crystal displays sandwich liquid crystal materials between two transparent electrodes, and when voltage is applied, the arrangement of liquid crystal molecules changes, affecting the propagation of light through the liquid crystal material and causing color changes. Clearly, this transparent electrode can only be achieved by coating a layer of indium tin oxide on the glass surface. Another typical example is solar cells, where one of the two electrodes must be transparent to allow light to enter the solar cell and be converted into electrical energy, thus indium tin oxide is again utilized.
Although indium tin oxide nearly perfectly combines the properties of conductivity and transparency, scientists have already begun searching for alternatives. But why? Firstly, indium is a very rare element, with its abundance in the Earth’s crust slightly higher than that of silver, and it is widely dispersed in the crust, so the available resources are not very rich. Some even predict that in a few decades, indium will be exhausted on Earth. In today’s rapidly developing electronics industry, the shortage of indium tin oxide resources has become increasingly prominent. Secondly, the current method of coating indium tin oxide onto glass or polymer surfaces mainly uses vacuum deposition technology, which requires expensive equipment and is not very efficient in production. The combination of resource scarcity and high production costs has led to a significant increase in the price of indium tin oxide in recent years, inevitably impacting the cost of electronic products. Additionally, indium tin oxide has a fatal flaw: it is relatively hard and brittle, lacking ductility, and is prone to cracking under external force, which may affect the lifespan of electronic products.
Due to these shortcomings of indium tin oxide, researchers have been dedicated to finding alternative materials in recent years. Two categories of alternative materials are particularly noteworthy: one is conductive polymers. Generally, polymer materials are insulators, but some polymers can possess certain conductive properties due to their special structure. The other is graphene, which has become very popular in recent years. As we know, the structure of graphite resembles a stack of paper, where each “sheet” consists of carbon atoms connected in a layered structure. If we break apart this stack of “paper,” we obtain individual layers, and this very thin material is called graphene. Like indium tin oxide, conductive polymers and graphene also possess good conductivity and transparency. These two materials are primarily composed of common elements such as carbon, hydrogen, and oxygen, making them virtually inexhaustible resources.
However, their advantages do not stop there. Firstly, both conductive polymers and graphene are much easier to process, requiring no expensive vacuum deposition equipment. We can dissolve or disperse them in water or other liquids and then cover the surface of glass or plastic with this “ink” just like printing books. Secondly, both conductive polymers and graphene have excellent ductility, able to withstand considerable bending, folding, or squeezing without breaking, which indium tin oxide cannot match. Replacing indium tin oxide with these materials could help achieve so-called flexible electronics, which would be a revolutionary breakthrough. Perhaps in the near future, our smartphones will no longer be kept in pockets or bags but directly sewn into clothing; and tablets or e-readers could be folded like handkerchiefs, opened when in use and folded away when not.
Whether it is conductive polymers, graphene, or other materials that can replace indium tin oxide, they all still have various issues that need to be resolved to different extents. However, with the continuous advancement of technology, it is possible that in the near future, indium tin oxide, which has made significant contributions to our electronic products, may indeed fade from our sight. Of course, we need not feel sad about its departure: “Out with the old, in with the new.” The day indium tin oxide is replaced by new transparent conductive materials will also be the day our electronic products undergo an upgrade.




