Follow Us By Clicking The Blue Text Above
Editor’s Note: In recent years, exploring shorter and more effective drug combinations for treating drug-sensitive tuberculosis (DS-TB) has become a significant trend in tuberculosis research, aimed at reducing adverse reactions, enhancing treatment adherence, and improving treatment efficiency and resource utilization. Previous studies such as Study 31/A5349, SHINE, TRUNCATE-TB have been reported. At the recently concluded 31st Conference on Retroviruses and Opportunistic Infections (CROI 2024), a groundbreaking abstract (Late breaking abstract) was selected for oral presentation, discussing the efficacy and safety of a 3-month regimen containing Clofazimine/Rifapentine for treating DS-TB (Abstract No: 164). “Infection Medical Line” invited Professor Lu Shuihua from Shenzhen Third People’s Hospital to form a team to interpret the progress of tuberculosis research, introducing and commenting on this study as follows.
Study Introduction
Abstract No: 164
Provisional Results From a 3-month Clofazimine/Rifapentine Containing Regimen for Drug-Sensitive TB
Initial Results of a 3-Month Regimen Containing Clofazimine/Rifapentine for Drug-Sensitive Tuberculosis
Background:
CLO-FAST (ACTG A5362; NCT04311502) is a randomized, controlled, open-label phase IIc clinical trial designed to evaluate the safety and efficacy of a 3-month regimen of Rifapentine (P, 1200mg daily) / Clofazimine (CFZ, 300mg daily for the first three weeks, then 100mg) (8w HPZEC/ 5w HPZC) compared to a 6-month standard treatment regimen (SOC), which was halted early due to lack of clinical efficacy. This report presents data results as of September 25, 2023, when the last participant completed the study treatment.
Methods:
Adults with DS-TB were recruited at six sites in Malawi, Zimbabwe, South Africa, India, and Haiti. Randomization was stratified by HIV status and advanced disease as determined by chest X-ray, into: Group 1 (13-week experimental regimen) receiving 3PHZEC; Group 2 (26-week SOC) receiving 2RHZE/4RH; Group C (PK subgroup without CFZ loading, then SOC) receiving 1PHZEC/1RHZE/4RH. The primary outcome of the study was the time to stable liquid culture conversion by week 12 in Groups 1 and 2 (efficacy) and the proportion of participants with ≥ grade 3 adverse events (AEs) by week 65 (safety). The cumulative proportion of adverse clinical/bacteriological outcomes in Groups 1 and 2 by week 65 was a key secondary outcome of the study.
Results:
Between June 2021 and April 2023, when the trial was terminated due to ineffectiveness based on DSMB recommendations, 58 of the planned 110 participants were included in Group 1, 31 of the planned 55 participants in Group 2, and 15 of the planned 20 participants in Group C. The majority of participants were male (79%), and 29% were HIV-positive (median baseline CD4+ cell count 265 cells/mm3, IQR 185-379 cells/mm3). Seventy-one percent of patients had radiological advanced tuberculosis, and 25% were 3+ smear positive at enrollment. The median study follow-up time (IQR) was 50.2 weeks (39.7, 57.6 weeks). By week 12, 89% (n=48) of participants in Group 1 and 90% (n=28) of participants in Group 2 achieved stable culture conversion (adjusted HR 1.17, 90%CI: 0.79 to 1.73, adjusted for baseline HIV status and presence of advanced disease). Influenced by changes in creatinine clearance rates in Group 1, the cumulative proportion of participants with ≥ grade 3 adverse events in Group 1 (46%) was higher than in Group 2 (16%) (difference 30%, 90%CI: 14-45%). The cumulative incidence of adverse outcomes in Groups 1 and 2 was 49% (95%CI: 34-67%) and 34% (95%CI: 19-58%) respectively (difference -15%, 95%CI: -41% to 11%). The table below describes the clinical/bacteriological adverse outcome events.
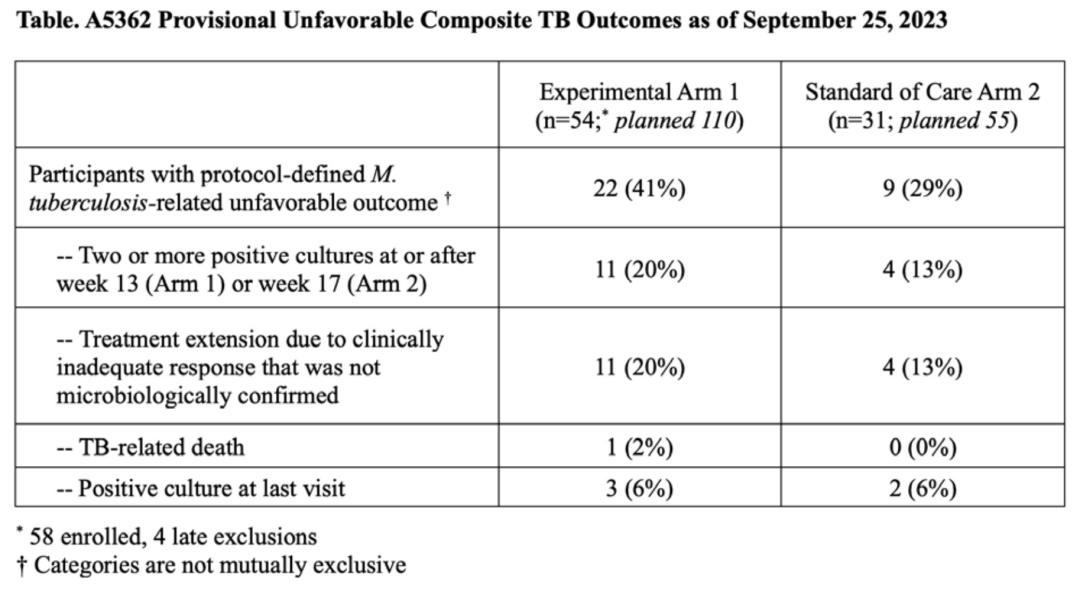
Conclusion:
The 13-week treatment regimen containing CFZ and Rifapentine showed poor efficacy for DS-TB. Despite a high culture conversion rate at week 12, the incidence of adverse events in the SOC group was also high.
Study Review
In recent years, a significant trend in tuberculosis research has been to explore shorter and more effective drug combinations for treating drug-sensitive tuberculosis (DS-TB). The key motivations and goals behind this trend include: (1) Enhancing patient adherence: Standard treatment regimens (HRZE) typically last 6 months or longer, placing a significant burden on patients and leading to treatment interruptions or incompleteness, which may result in treatment failure and the development of drug resistance. Shorter regimens aim to alleviate this burden and improve treatment completion rates. (2) Reducing adverse reactions: Long-term use of anti-tuberculosis drugs may increase the risk of adverse reactions, particularly hepatotoxicity and other side effects. Shorter regimens are expected to mitigate these risks. (3) Improving treatment efficiency and resource utilization: Shorter treatment regimens can enhance the efficiency of healthcare resource usage, which is particularly significant in resource-limited settings for public health improvement.
Successful cases of shorter treatment regimen research include: (1) Study 31/A5349: By using high-dose Rifapentine instead of Rifampicin and replacing Ethambutol with Moxifloxacin, the treatment duration was shortened to 4 months. (2) SHINE study: For children and adolescents with milder conditions, the standard regimen was shortened to 4 months. (3) TRUNCATE-TB study: Using Bedaquiline + Linezolid instead of Rifampicin for a 2-month intensive treatment, or adjusting to 3 months based on treatment response, with a total duration reduced to 86 days if results were unsatisfactory. These three studies were published in 2021, 2022, and 2023 respectively, representing significant progress in shorter treatment regimens.
The Clo-Fast study was conducted concurrently, attempting to explore new treatment regimens by adding Clofazimine to the standard regimen and replacing Rifampicin with high-dose Rifapentine. Clofazimine, originally used for leprosy treatment, has recently gained prominence in tuberculosis treatment by disrupting the cell membrane and electron transport chain of Mycobacterium tuberculosis, proving effective against multidrug-resistant and extensively drug-resistant strains, and is one of the WHO-recommended drugs for treating multidrug-resistant tuberculosis; its main side effects include skin discoloration, gastrointestinal discomfort, and QT interval prolongation. In recent years, the anti-tuberculosis effects of Rifapentine have also been emphasized, with most studies recommending higher doses. Higher doses of Rifapentine may be more effective in shorter treatment regimens.
However, the Clo-Fast study did not achieve success, possibly due to: (1) Insufficient efficacy of the drug regimen: The 3-month regimen (including high-dose Rifapentine and Clofazimine) failed to achieve the same treatment effect as the 6-month standard regimen, despite similar stable culture conversion rates at week 12, the efficacy of shorter regimens for treating DS-TB was inadequate. (2) Higher incidence of adverse events: The proportion of adverse events (especially those related to changes in creatinine clearance) in the experimental group was higher than in the standard treatment group, possibly indicating that the safety of the shorter regimen was inferior to that of standard treatment, impacting overall treatment outcomes. Clofazimine is primarily excreted through feces, with only a small portion excreted through urine, and its excretion may not be significantly affected in patients with renal dysfunction, typically not requiring dose adjustments. The reasons for renal adverse events in the experimental group still require further analysis, with a need to be cautious of the renal toxicity brought by high-dose Rifapentine or drug interactions in the regimen leading to renal toxicity. (3) Higher probability of adverse outcomes: The cumulative probability of adverse outcomes (clinical/bacteriological) in the experimental group was higher than in the standard treatment group, possibly indicating that the shorter regimen did not effectively eliminate the infection or prevent relapse. Compared to the TRUNCATE-TB study, the treatment regimen in the Clo-Fast study may have been less effective; the former used two WHO Group A recommended drugs (Bedaquiline and Linezolid) and did not use Rifamycins, while the latter only used one WHO Group B recommended drug (Clofazimine) and employed high-dose Rifapentine. (4) Insufficient sample size and early termination: The study was terminated early due to not meeting expected outcomes, with participant numbers not reaching the planned sample size, potentially limiting the statistical power of the study results and reliability of conclusions. (5) Diversity of population and disease: The study spanned different countries, with participants having various demographic characteristics and disease statuses (such as HIV infection and severity of tuberculosis), this diversity may affect treatment response and the occurrence of adverse events.
In summary, the Clo-Fast study was innovative in attempting to shorten the treatment duration for DS-TB, but ultimately failed to meet expected efficacy and safety targets. The results of this study emphasize the need to consider both efficacy and safety, as well as individual patient differences when developing new short-course treatment regimens. Additionally, the Clo-Fast study also highlighted the challenges faced in conducting such trials, including selecting appropriate drug combinations and ensuring sufficient sample sizes to achieve statistically meaningful results.
Despite the study’s lack of success, its experiences and lessons are crucial for future tuberculosis treatment research. It prompts us to reflect on current treatment strategies, explore more innovative approaches, and adopt more comprehensive study designs in future clinical trials. For the future of tuberculosis treatment, ongoing research, innovation, and adaptive adjustments will be key.
Recommended Reads
|
1 |
Shenzhen Third People’s Hospital Tuberculosis Window丨First Report on New Vaccine for Preventing Tuberculosis Recurrence in RCT Study |
|
2 |
Shenzhen Third People’s Hospital Tuberculosis Window丨PAN-TB Treatment Trends and Mid-term Report Review of QDB Study |
|
3 |
Shenzhen Third People’s Hospital Tuberculosis Window丨Hormonal Treatment Does Not Improve Survival in HIV-Related Tuberculous Meningitis Patients, Further Optimization of Beneficiary Population Screening Needed |

Professor Lu Shuihua
Professor, Chief Physician, Associate Professor, Doctoral Supervisor
Deputy Director of the National Clinical Research Center for Infectious Diseases, Director of the Pulmonary Medicine Department of Shenzhen Third People’s Hospital
Positions held: Chair of the Tuberculosis Branch of the Chinese Medical Association, Member of the WHO Global Working Group on Tuberculosis in Children and Adolescents, Chair of the School and Children Tuberculosis Branch of the China Anti-Tuberculosis Association, Honorary Chair of the Tuberculosis Branch of the Shanghai Medical Association, Chair of the Tuberculosis Branch of the Guangdong Medical Association, Recipient of the Second National Famous Doctor Award, Top Ten Physicians in Shanghai, Head of the National Major Project on Infectious Diseases during the 13th Five-Year Plan, Principal Investigator of Major Projects of the National Natural Science Foundation, Member of the Expert Group for Influenza Medical Treatment of the National Health Commission, Reviewer Expert for the National Natural Science Foundation, Reviewer Expert for New Drug Evaluation of the National Medical Products Administration, Reviewer Expert for Medical Devices of the National Medical Products Administration, Expert in Medical Appraisal of the Chinese Medical Association, and Associate Editor, Editorial Board Member, and Reviewer Expert for multiple journals.
Long-term engagement in research on the pathogenesis of TB, vaccine and diagnostic technology development, epidemiology, and clinical trials of new drugs: Conducting large-scale population cohort studies to clarify the Mtb infection rate and BCG protection efficiency among university students in China; constructing and implementing “layered solutions for tuberculosis infection immune diagnosis,” leading the first clinical trial and market approval of the first class I new TB drug (EC) in 40 years in China; actively exploring new strategies for TB prevention and control, establishing and promoting early screening and precise intervention systems for latent tuberculosis infection; proposing an “integrated comprehensive prevention and control strategy” to contribute China’s original theories and technological products to “End TB.” Leading 11 national, provincial, and municipal scientific research projects, including the National Major Project on Infectious Diseases during the 13th Five-Year Plan, Major New Drug Creation Project, and Major Projects of the National Natural Science Foundation, with a total research funding exceeding 72.6 million yuan. Published 73 academic papers in top journals such as NEJM, Lancet, PNAS, organized and authored 3 guidelines and expert consensus, and edited 1 monograph. Related achievements have won the First Prize of the “China Anti-Tuberculosis Association Science and Technology Award” and the Third Prize of the “Shanghai Medical Science and Technology Award,” and have been cited multiple times in WHO guidelines.
Fu Liang
Attending Physician of the Second Department of Pulmonary Diseases, National Clinical Research Center for Infectious Diseases / Shenzhen Third People’s Hospital
Master’s in Respiratory Medicine from Southern Medical University, PhD in Tuberculosis from Capital Medical University. Personnel in the “Global Health Talent Reserve” of the National Health Commission. Main research directions include all-oral short-course treatment for multidrug-resistant tuberculosis, new treatment regimens for non-tuberculous mycobacterial disease, rapid non-invasive diagnosis of tuberculosis, etc. Member of the Non-Tuberculous Mycobacterial Disease Branch of the China Anti-Tuberculosis Association; Member of the Clinical Trials Branch of the China Anti-Tuberculosis Association. Editorial board member of the tuberculosis channel of Medical Reference News. Participated in writing 1 domestic tuberculosis guideline, 4 consensus documents, and 6 books. Published 15 articles in Chinese and 1 in English. Led 1 municipal project and participated in 3 municipal projects, 1 provincial project, and 1 national project.
Master’s in Respiratory Medicine from Southern Medical University, PhD in Tuberculosis from Capital Medical University. Personnel in the “Global Health Talent Reserve” of the National Health Commission. Member of the Non-Tuberculous Mycobacterial Disease Branch and Clinical Trials Branch of the China Anti-Tuberculosis Association; Member of the Scientific Research Committee of the Tuberculosis Branch of the Chinese Medical Association. Editorial board member of Emerging Infectious Diseases Journal. Participated in writing 1 guideline, 5 consensus documents, and 6 books. Designed and led a nationwide multicenter study on multidrug-resistant tuberculosis treatment (ongoing). Received 1.4 million yuan in research funding over the past two years.
Statement
All original articles signed belong to “Infection Medical Line,” sharing and reprinting are welcome (please leave a message in the background for permission). This article is for the reference of medical and health professionals to understand the latest medical information and does not represent the views of this platform. This information cannot replace professional medical guidance in any way and should not be considered as medical advice. If this information is used for purposes other than information, this site and the author do not bear any related responsibilities.
