[Neuroscience Cutting-Edge Technology Training Series] See details at the end
[Content Promotion, Lectures/Reports/Courses and Other Academic Collaborations] Please contact WeChat: Wang_Sizhen
Editor | Wang Sizhen

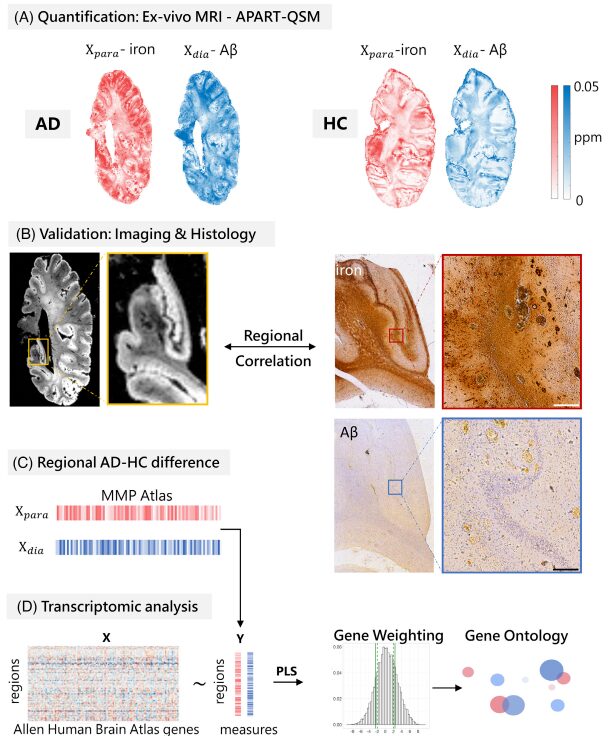
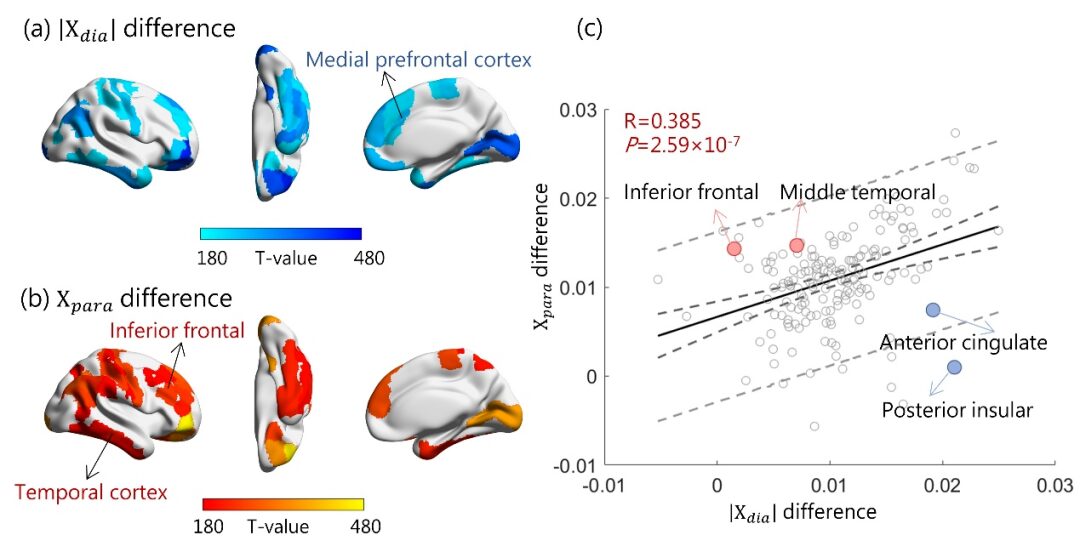
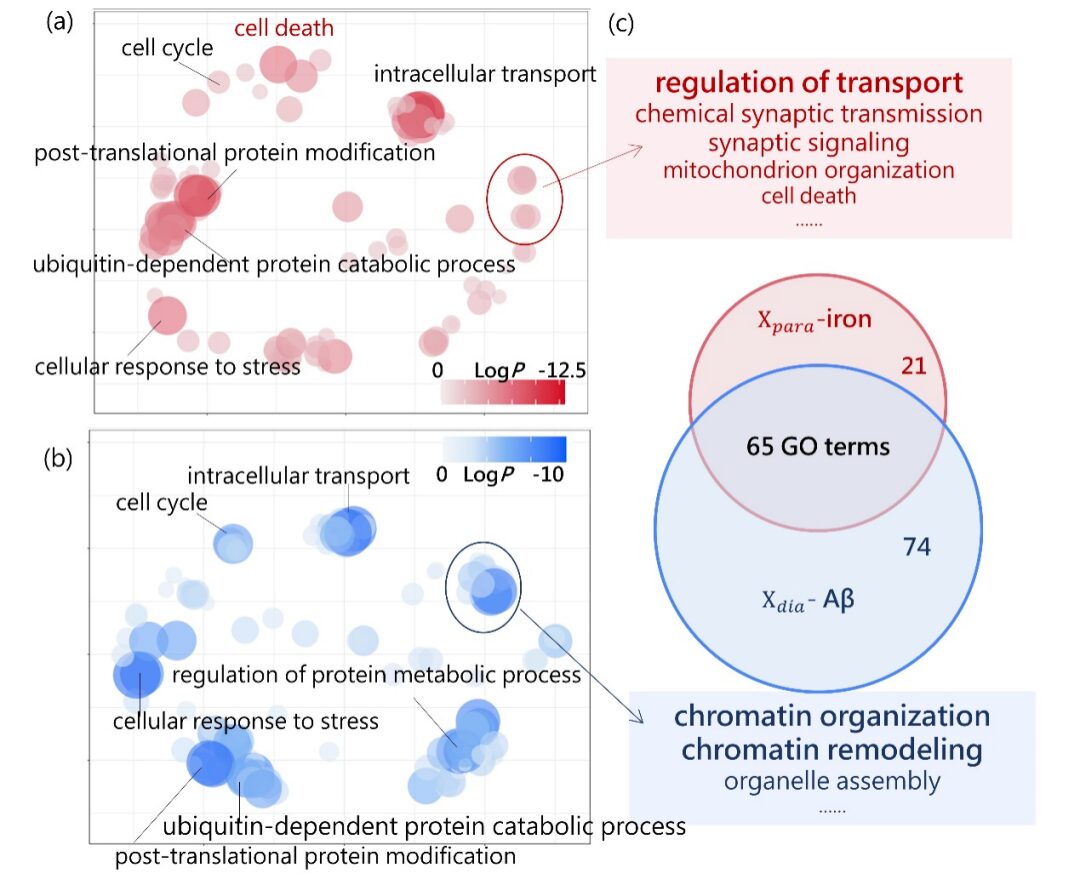
This study simultaneously excavates the rich information contained in post-mortem human brain specimens from both macro imaging and micro pathology scales and incorporates transcriptomic analysis, analyzing the gene expression characteristics behind brain Aβ protein and iron deposition, which is of great significance for deepening the pathological research of AD. It is worth emphasizing that the innovative 7T ultra-high field magnetic resonance imaging technology can analyze the changes in diamagnetic and paramagnetic susceptibility in quantitative magnetic susceptibility signals, and shows a close correlation with the pathological progression of AD, suggesting the potential clinical application value of quantitatively assessing the two magnetic susceptibility characteristics, which may provide new imaging indicators for the early clinical diagnosis of AD, offering better cost-effectiveness compared to existing PET molecular imaging diagnosis, benefiting a wider patient population.
Reprint Notice::Non“Logical Neuroscience” team original manuscripts and (or) invited manuscripts, this content is authorized for reprint, copyright belongs to the original author and (or) original unit..
[Optogenetics and Genetic Encoding Calcium Probes and Neurotransmitter Probe Working Principles and Applications] and [In Vivo Imaging Techniques in Neuroscience Research Basics and Applications] (Issue 9), time March 22-24, 2025 (Saturday to Monday); location Nanjing.
[Patch-Clamp Recording System Operation and Application] (Issue 7). Time To be determined; location Nantong.
[Neuroscience Cutting-Edge Technology Integration: When In Vivo Electrophysiology Meets Optogenetics] (Issue 2), time To be determined (Saturday to Monday); location Nanjing.
[National Frontier Tissue Transparency and Three-Dimensional Imaging Theory and Skills Training Course] (Issue 2), time To be determined; location Wuhan.
[Disease Research and Animal Behavior Series]
[Latest Research Progress and Hotspots of Alzheimer’s Disease and Experimental Design Ideas and Data Analysis of Animal Behavior] (Issue 2). Time March 23, 2025 (Sunday), location Online.
“Logical Neuroscience” WeChat Group: Literature Study
