
Research Background

Three-dimensional (3D) bioprinting of liver tissue models provides a new strategy for the treatment of liver failure. However, current research on bioprinting liver tissue models mainly relies on traditional single-cell bioprinting techniques, where individual functional hepatocytes are dispersed and isolated in hydrogels, leading to insufficient cell function and poor therapeutic outcomes.
Recently, the research teams led by Wei Sun and Yuan Pang from Tsinghua University, along with Hongkui Deng’s team from Peking University, published a high-level paper titled “Bioprinting functional hepatocyte organoids derived from human chemically induced pluripotent stem cells to treat liver failure” in the internationally renowned journal Gut. This study utilized functional hepatic organoids (hCiPSC-HOs) derived from human chemically induced pluripotent stem cells (hCiPSCs) and employed cell spheroid bioprinting technology to construct liver tissue models (3DP-HOs), which were evaluated for their liver-specific functions both in vitro and in vivo (Figure 1). The study found that compared to single-cell bioprinting models, 3DP-HOs exhibited enhanced cell viability, with gene expression profiles closely resembling those of hCiPSC-HOs, maintaining liver-specific functions. Furthermore, the implantation of 3DP-HOs significantly improved the survival rates of carbon tetrachloride (CCl4)-induced acute liver failure and Fah knockout mouse models, significantly reducing liver injury, inflammation, and fibrosis markers, while promoting liver regeneration and the expression of genes related to biological function.
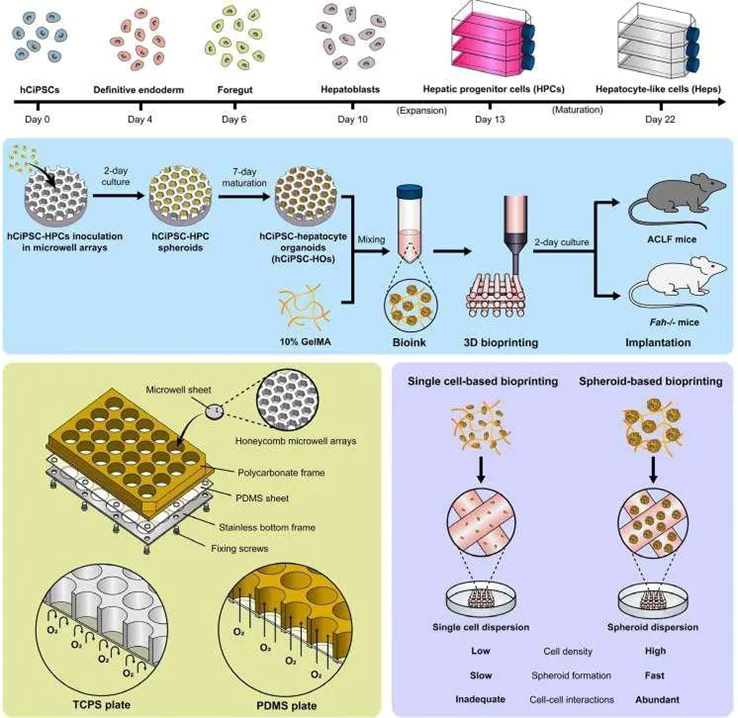
Figure 1 Experimental process and research focus schematic (adapted from reference)

Research Results

1. Preparation and Liver Function Characteristics of hCiPSC-HOs
The research team first utilized hCiPSCs as the cell source and differentiated them into hepatic organoids (HOs) through a specific induction differentiation protocol. During the differentiation process, the researchers employed an oxygen-permeable microporous array device to enhance oxygen supply, maintaining high cell viability and promoting the maturation of HOs. This microporous array device provides sufficient oxygen, simulating the microenvironment of the liver in vivo, thereby improving the differentiation efficiency and function of the cells. The results showed that hCiPSC-HOs exhibited characteristics similar to primary human hepatocytes (PHHs) in terms of gene expression, protein synthesis, and metabolic function (Figure 2). For example, hCiPSC-HOs highly expressed liver-specific genes such as HNF4A, CYP3A4, CPS1, and F5, and demonstrated high albumin secretion and urea synthesis capabilities, which are important indicators of liver function. Additionally, hCiPSC-HOs also displayed good drug metabolism and bile acid synthesis capabilities, indicating mature hepatocyte functionality.
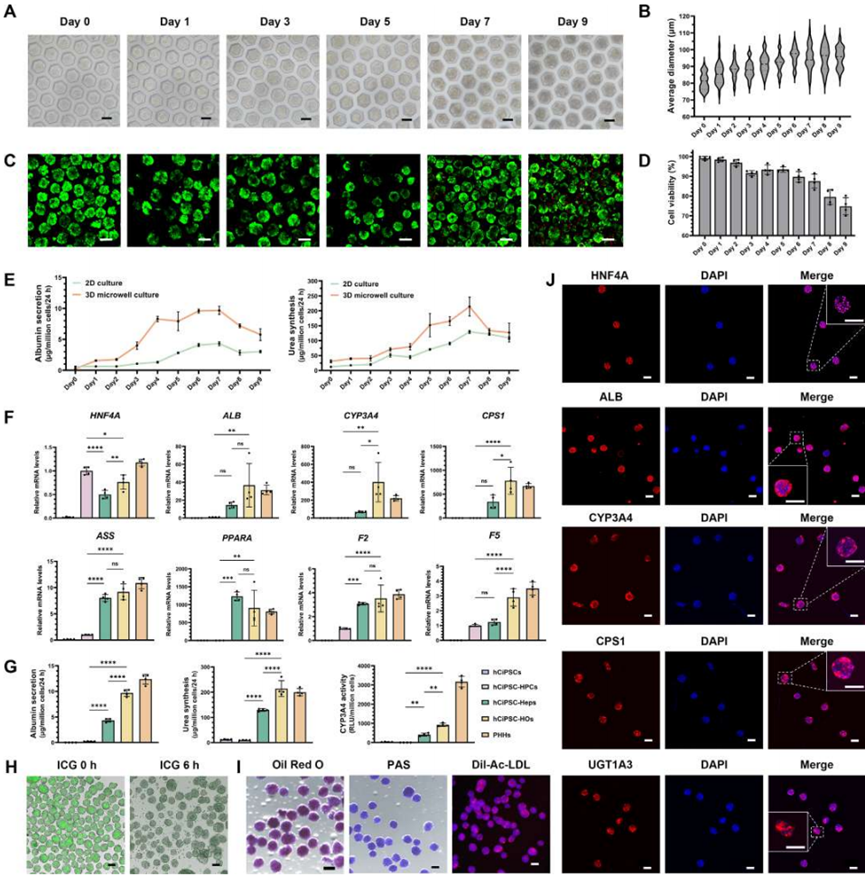
Figure 2 Generation of high-functionality hCiPSC-HOs using oxygen-permeable microporous arrays (adapted from reference)
2. Construction and Advantages of 3DP-HOs
The differentiated hCiPSC-HOs were mixed with 10% GelMA (methacrylated gelatin) precursor to prepare bioink. GelMA is a commonly used bioprinting material with good biocompatibility and mechanical properties, capable of rapidly curing at room temperature to form stable three-dimensional structures. By optimizing parameters such as cell density, printing speed, and temperature, the researchers successfully printed hCiPSC-HOs into predetermined three-dimensional structures. Compared to traditional single-cell bioprinting methods, spheroid bioprinting technology allows cells to maintain closer interactions in a three-dimensional environment, better simulating the tissue structure of natural liver. 3DP-HOs exhibited significant advantages in cell viability, structural stability, and functional maintenance. 3DP-HOs rapidly restored cell viability after printing and maintained stable liver-specific functions, such as albumin secretion, urea synthesis, and drug metabolism during in vitro culture (Figure 3). The gene transcriptome expression of liver organoids before and after printing showed similar profiles, with some liver-specific functional genes expressing levels close to those of primary human hepatocytes (Figure 4).
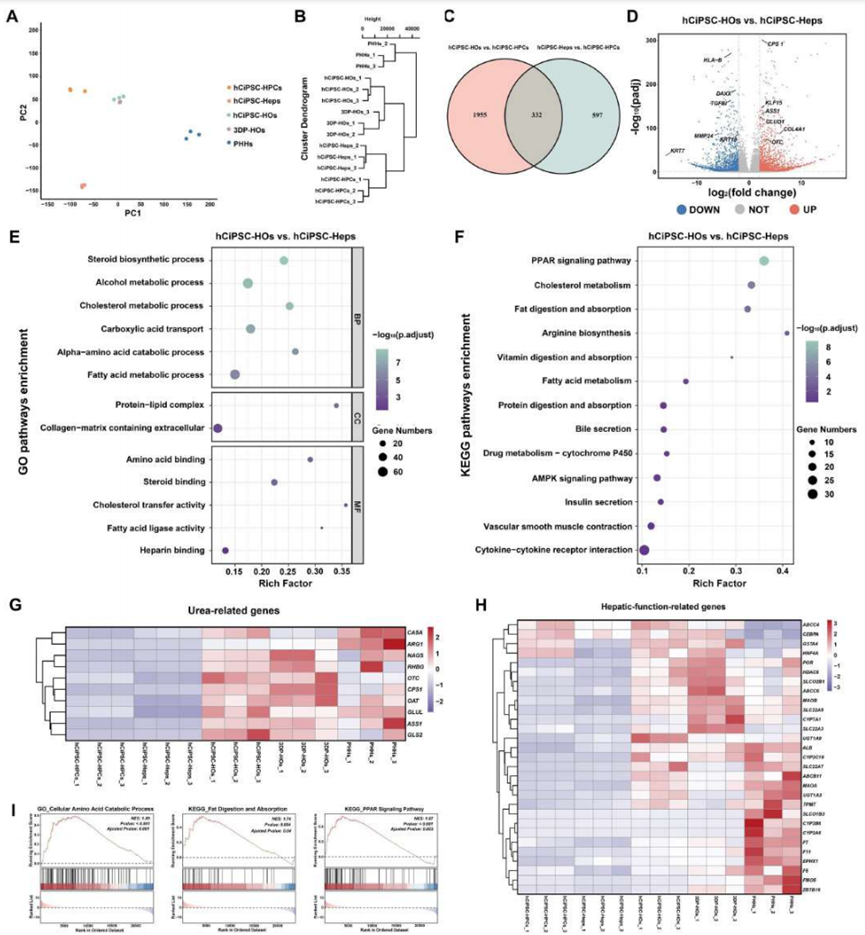
Figure 3 Spheroid-based liver tissue model bioprinting ensures high cell viability and biological function of hCiPSC-HOs (adapted from reference)

Figure 4 RNA sequencing shows similar gene expression profiles between hCiPSC-HOs and 3DP-HOs, with enhanced liver function (adapted from reference)
3. In Vivo Transplantation of 3DP-HOs for Liver Failure Treatment
In in vivo transplantation treatments for two mouse models of liver failure induced by CCl4 (Figure 5) and Fah gene deficiency (Figure 6), 3DP-HOs significantly improved mouse survival rates, reduced liver injury markers, decreased liver inflammation levels, and human albumin was detected in mouse serum, demonstrating that 3DP-HOs possess good synthetic secretion functions in vivo.
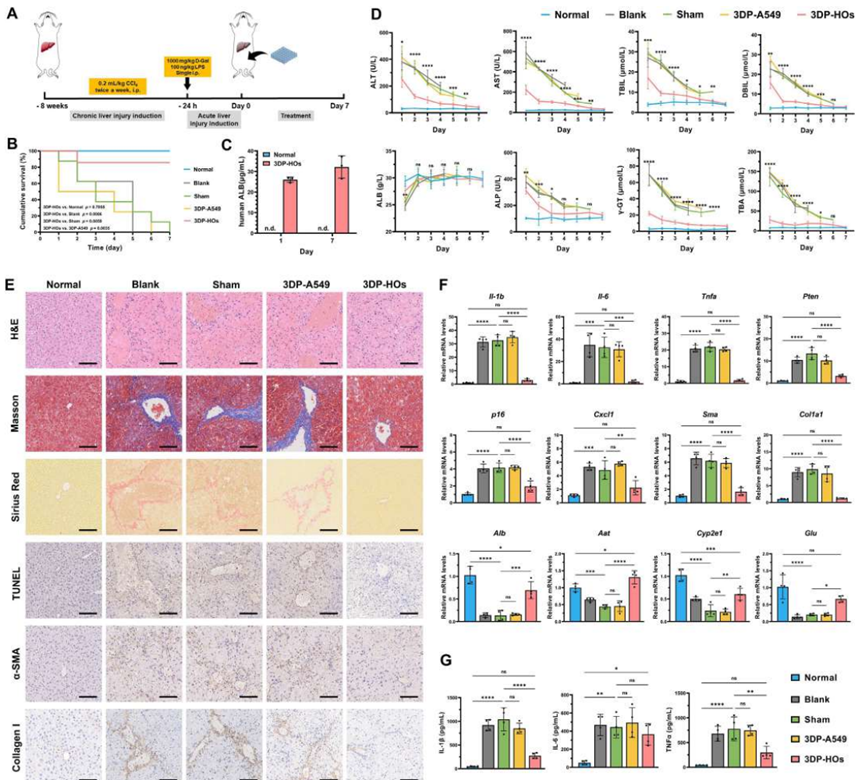
Figure 5 3DP-HOs implantation rescues CCl4-induced acute liver failure mice (adapted from reference)

Figure 6 3DP-HOs implantation rescues Fah-deficient liver failure mice (adapted from reference)
4. Long-term Function Maintenance and Vascularization of 3DP-HOs in Vivo
In long-term in vivo transplantation experiments, 3DP-HOs exhibited good biocompatibility and stability (Figure 7). After 60 days of transplantation, 3DP-HOs maintained intact structures and high cell viability. The researchers observed significant vascular connections formed between 3DP-HOs and the host, which not only provided sufficient oxygen and nutrients to the transplanted tissue but also facilitated the removal of harmful substances. This vascularization phenomenon indicates that 3DP-HOs can effectively integrate with the host’s physiological system, providing important support for their long-term functional maintenance.

Figure 7 During the treatment of Fah-deficient liver failure mice, 3DP-HOs promote vascularization and maintain high cellular biological function (adapted from reference)

Research Conclusion

This study successfully developed a novel method for constructing liver tissue models based on hCiPSC-derived HOs and spheroid bioprinting technology, achieving significant therapeutic effects in mouse models of liver failure. The results indicate that 3DP-HOs possess good biocompatibility, stability, and liver-specific functions, effectively improving the survival rates and liver function of liver failure mice. This research provides new strategies and methods for the clinical treatment of liver failure, with significant scientific significance and application prospects.
In the future, the research team will continue to optimize 3D bioprinting technology and the construction methods of liver tissue models to further enhance their feasibility and safety for clinical applications. They will also explore the potential of applying this method to other types of liver diseases, bringing hope to more patients.
References: Li G, He J, Shi J, Li X, Liu L, Ge X, Chen W, Jia J, Wang J, Yin M, Sakai Y, Sun W, Deng H, Pang Y. Bioprinting functional hepatocyte organoids derived from human chemically induced pluripotent stem cells to treat liver failure. Gut. 2025 Mar 3:gutjnl-2024-333885.