Due to the involvement of NTRK gene fusions in various tumors, their occurrence is rare, and the standard therapies (SoC) and prognoses for different tumors vary. Conducting clinical trials of TRK inhibitors using the “gold standard” randomized controlled trials (RCTs) is often impractical. Currently, clinical trials for TRK inhibitors mostly employ single-arm basket study designs to provide rapid and effective treatment options for patients with NTRK gene fusions who previously had no effective medications available. However, single-arm trials cannot directly provide evidence for efficacy comparisons between different drugs, and addressing this limitation has been a research focus since the market introduction of TRK inhibitors. At this year’s ASCO conference, Larotrectinib was prominently featured in the VICTORIA study[1], which, with the aid of high-quality study design and statistical methods, compared Larotrectinib under single-arm basket clinical trial conditions with non-TRK inhibitor treatments in the real world, further demonstrating Larotrectinib’s irreplaceable role as a TRK inhibitor for patients with NTRK gene fusion malignant tumors and providing effective evidence for comparison with non-TRK inhibitors. Based on this, we invited Professor Chen Pingyan from Southern Medical University and Professor Chen Yonghui from Peking Union Medical College Hospital to interpret the study and explore the important value and development prospects of Larotrectinib in the field of pan-tumor treatment.
Invited Experts
Doctoral supervisor, postdoctoral cooperative mentor
Former Director of the Department of Biostatistics, Southern Medical University (1997-2019)
Executive Director of Hainan Real World Data Research Institute
Founded the first Department of Biostatistics in China (2005) and pioneered the establishment of a biostatistics undergraduate program (science, 2005)
Former Chair of the Biostatistics Branch of the Chinese Preventive Medicine Association (2014-2021)
Founder/President of the Guangdong Biostatistics Society
Vice President of the Chinese Branch of the International Biostatistics Society
Published over 300 papers, including more than 110 SCI papers, in journals such as SMMR, SIM, NEJM, JAMA, JCO, JAMA-IN, JAMA-SUR, ERJ, Critical Care, EClinicalMedicine, JASN, Radiology, Chest, Sleep, Hearts, JACC Cardiovasc Interv, etc.
Editor of the widely influential “SPSS Statistical Software Application Tutorial” series of graduate textbooks.
Master of Medicine, currently Deputy Chief Physician of the Nuclear Medicine Department at Peking Union Medical College Hospital, Associate Professor, Master’s supervisor, member of the 68Ga Imaging and Precision Therapy Committee of the Nuclear Medicine Branch of the Chinese Medical Association, member of the Treatment Group of the Nuclear Medicine Branch of the Chinese Medical Association, standing committee member of the Thyroid Committee of the Beijing Endocrine and Metabolic Disease Society, member of the Radiopharmaceutical Therapy Group of the Nuclear Medicine Branch of the Beijing Medical Association. Since 2003, engaged in clinical, teaching, and research work in the Nuclear Medicine Department at Peking Union Medical College Hospital, specializing in integrated diagnosis and treatment of thyroid diseases such as 131I treatment for hyperthyroidism, differentiated thyroid cancer, neuroendocrine tumors, and advanced prostate cancer. Participated in multiple clinical research projects and received the third prize of the Chinese Medical Science and Technology Award in 2010 for the project “Establishment and Related Applications of the Comprehensive Diagnosis and Management Cooperation System for Hemophilia in Beijing.”
2024 ASCO Research Update

Research Title: VICTORIA Study – Real World Data Comparison of Larotrectinib vs Non-TRK Inhibitor Treatment in Patients with NTRK Gene Fusion Malignant Tumors Research Overview: The study included adult patients with positive NTRK gene fusions in non-small cell lung cancer (NSCLC), colorectal cancer (CRC), soft tissue sarcoma, thyroid cancer, or salivary gland cancer. The study employed precise matching methods to compare the efficacy outcomes of patients with NTRK gene fusion malignant tumors receiving Larotrectinib treatment (experimental group) in three clinical trials (NCT02122913, NCT02576431, NCT02637687) with patients receiving non-TRK inhibitor treatment (external control group [C1]) in a real-world (RW) environment (see Figure 1 for study design).
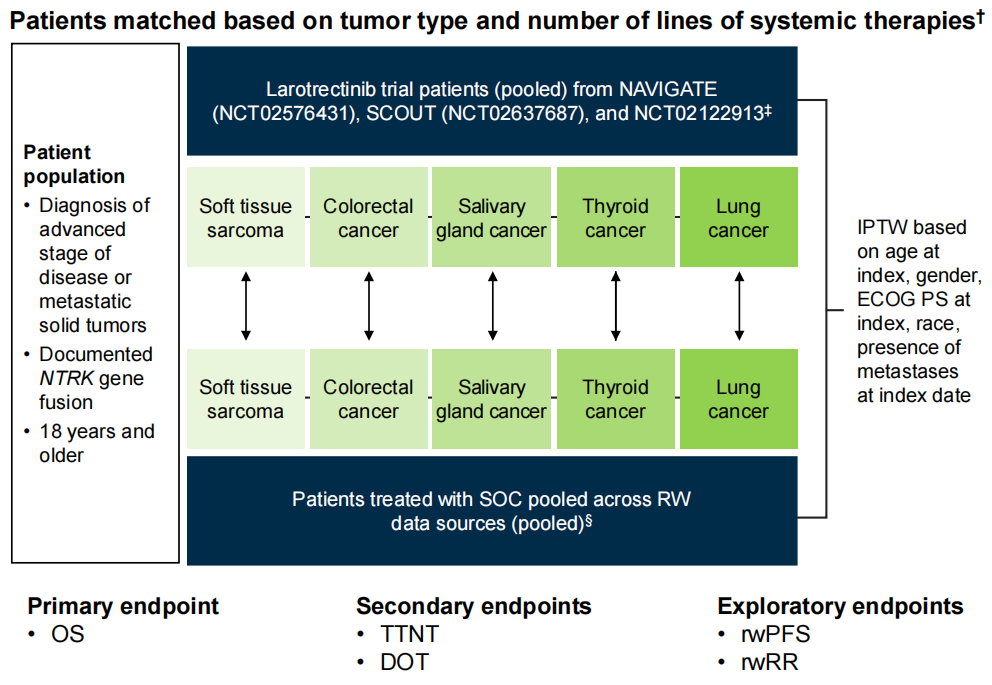 Figure 1 Study Design
Figure 1 Study Design
The study included 164 precisely matched patients with NTRK gene fusion malignant tumors, with 82 patients in the Larotrectinib group and 82 patients in the external control group. Patients receiving Larotrectinib treatment were precisely matched with control group patients based on tumor type and treatment line, and the indicated treatment line for control group patients was determined. A propensity score (weighted) model was used to balance key baseline characteristics between the two groups of patients, and the final propensity score model included five variables: gender, race, age, ECOG PS, and whether metastasis was present at the time of indication date. After weighted matching, baseline characteristics between the two groups of patients were balanced. The treatment conditions for the control group were as follows: chemotherapy (49%), non-TRK inhibitor small molecule targeted therapy (27%), chemotherapy + non-TRK inhibitor non-small molecule targeted therapy (11%), or immune checkpoint inhibitor treatment (10%) (see Table 1 for baseline characteristics of the two groups of patients).
Table 1 Key Baseline Characteristics of Enrolled Patients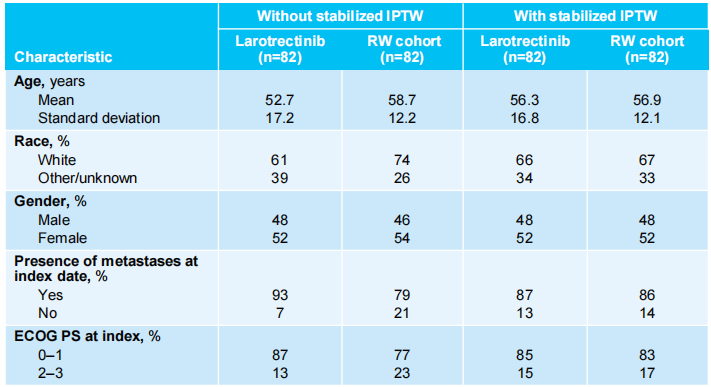
Key Results:
-
In the weighted analysis, the experimental group had a longer OS compared to the control group (median not reached vs 37.2 months; HR 0.44 [95% CI 0.23–0.83]), and the results were robust after thorough sensitivity analyses.
-
In the weighted analysis, the median treatment duration (DOT) in the experimental group was 30.8 vs 3.4 months, HR 0.23 [95% CI 0.15–0.33]; the time to next treatment (TNT) median was not reached vs 10.6 months, HR 0.22 [95% CI 0.13–0.38], both longer than the control group.
-
In the weighted analysis, the experimental group had a longer progression-free survival (PFS) compared to the control group, whether assessed by radiologists (median 36.8 vs 5.2 months; HR 0.29 [95% CI 0.18–0.46]) or oncologists (median 36.8 vs 5.9 months; HR 0.33 [95% CI 0.20–0.53]).
-
The results of the unweighted analysis were consistent with the weighted analysis regarding DOT, TTNT, and PFS.
The response rate (RR) of the experimental group (73.8% [95% CI 62.7–83.0]) was higher than that of the control group (53.8% [95% CI 37.2–69.9]).
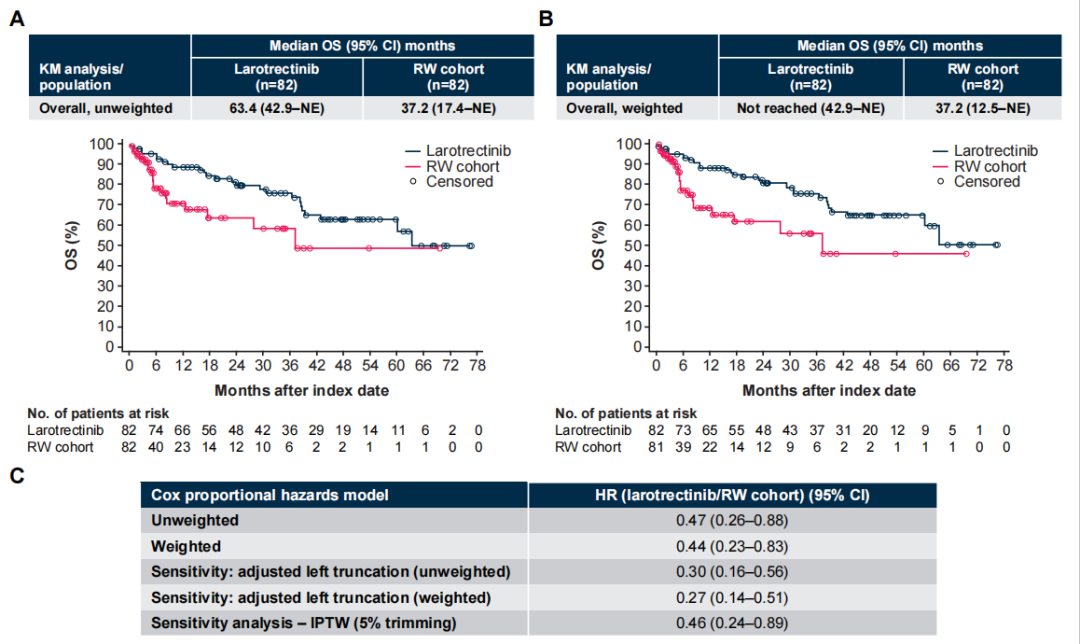 Figure 2 Unweighted (A) and Weighted (B) OS and Risk Ratio (C)
Figure 2 Unweighted (A) and Weighted (B) OS and Risk Ratio (C)
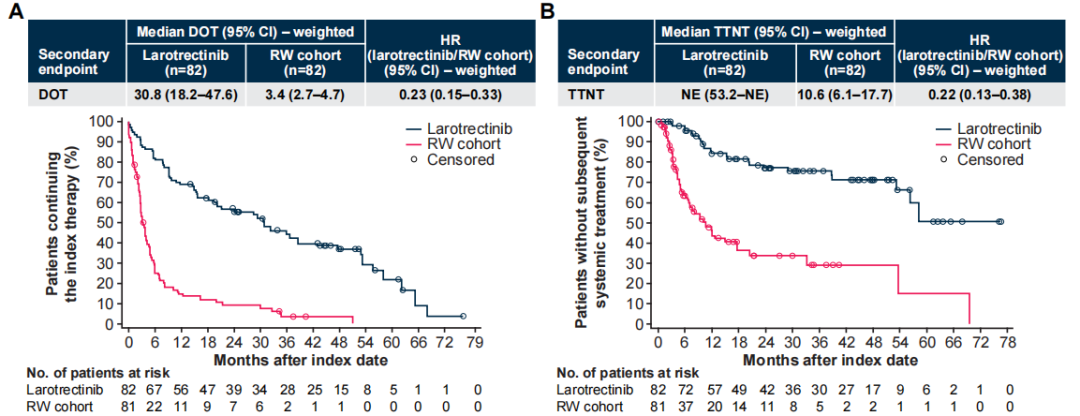 Figure 3 Weighted DOT (A) and TNT (B)
Figure 3 Weighted DOT (A) and TNT (B)
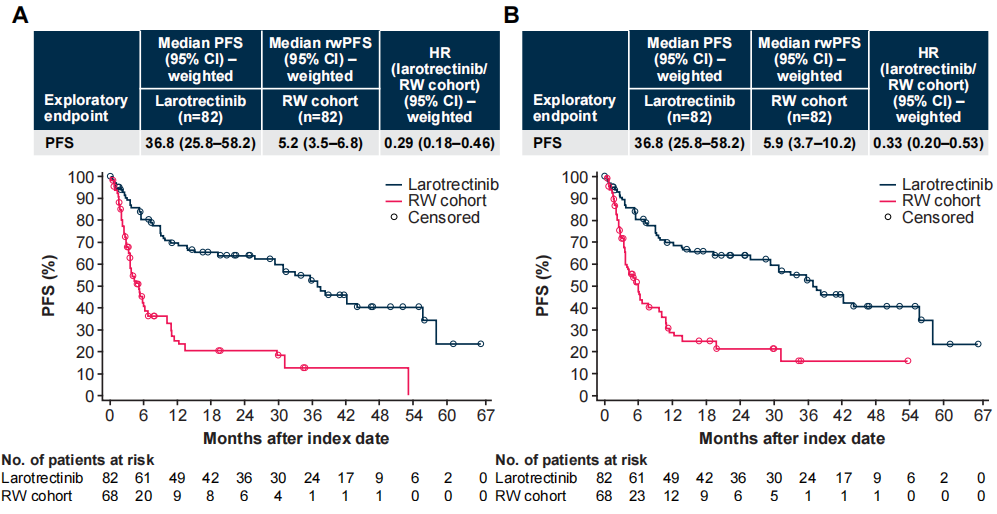 Figure 4 Weighted PFS and rwPFS, Radiologist Assessment (A) vs Oncologist Assessment (B)
Figure 4 Weighted PFS and rwPFS, Radiologist Assessment (A) vs Oncologist Assessment (B)
ASCO Expert Insights
Professor Chen Pingyan: High-quality study design and statistical methods bring possibilities for efficacy comparisons of rare target single-arm trial drugs.
In the clinical development of new drugs, RCTs are widely regarded as the gold standard for assessing and comparing intervention effects. However, in certain cases, feasibility is poor, such as when there are significant ethical risks, the diseases studied are life-threatening, refractory, and untreatable, or when the patient population is very small and rare diseases. Especially for targeted drugs like the TRK inhibitor Larotrectinib, which targets rare mutations, using single-arm basket studies is a superior design for market registration, where selecting appropriate external controls is crucial. Studies using external controls require careful consideration of controlling for confounding and bias. Propensity score matching and inverse probability weighting (IPTW) are commonly used methods for bias control. Generally, using these methods for correction can ensure that the baseline characteristics distribution between the two groups is consistent, ensuring comparability. In recent years, these methods have received increasing attention from clinical experts. Let’s look at a 2020 article in JCO, which studied the survival outcomes of patients with NTRK gene fusion malignant tumors receiving Larotrectinib versus standard treatment (SoC), employing a matching-adjusted indirect comparison (MAIC) method using propensity score matching. The data from three single-arm clinical trials of Larotrectinib (NCT02122913, NCT02637687, and NCT02576431) were compared with external controls constructed from real-world data of SoC in the Flatiron Health/Foundation Medicine database. This study ultimately included 85 patients receiving Larotrectinib treatment and 28 patients receiving SoC, with the matched adjusted analysis results showing that Larotrectinib could reduce the risk of death by 78% (HR 0.22, p=0.001), with median OS of 39.7 months and 10.2 months for the two groups. Compared to SoC, Larotrectinib treatment significantly extended the OS of patients with TRK fusion malignant tumors. More importantly, with extended follow-up time, the advantages of Larotrectinib treatment also further expanded, as shown in the figure below.
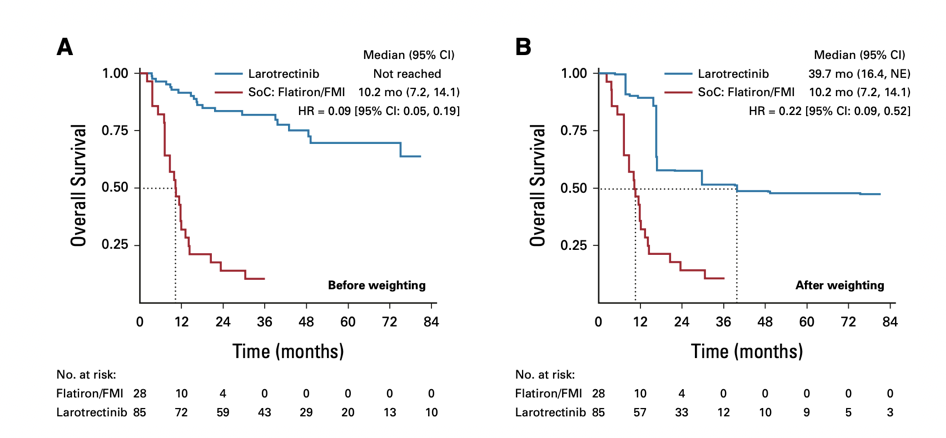
Figure 5 Unweighted (A) and Weighted (B) OS Results
This year’s ASCO announced VICTORIA study also used a similar strategy and analysis method as this JCO literature, employing real-world data as external controls, and with a larger sample size, each with 82 patients in the Larotrectinib group and the non-TRK inhibitor external control group based on real-world data after matching. From the results, Larotrectinib demonstrated a very significant advantage compared to non-TRK inhibitors. Therefore, in clinical research, for patient populations that are very limited, involve a wide variety of tumor types, and have varying control treatments for each tumor type, if high-quality real-world data is available, it can be used as external controls. By employing appropriate statistical analysis methods, such as propensity score matching or inverse probability weighting, it can provide a feasible path and strong support for single-arm trials to obtain key research support for new drug approvals, helping to inform clinical decisions and optimize treatment practices.
Professor Chen Yonghui: Targeting with precision, fully leveraging the advantages of Larotrectinib’s targeted therapy.
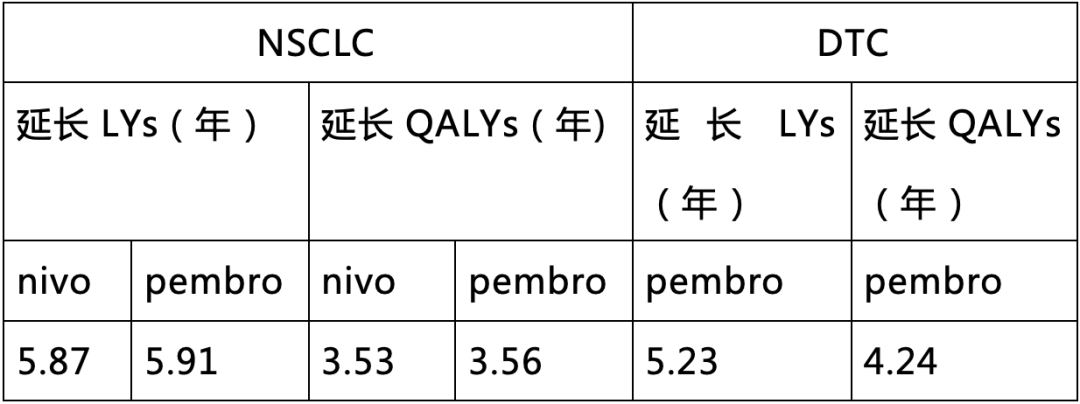
As a rare mutation, NTRK gene fusion has received increasing attention in recent years. NTRK gene fusions can be found in various adult and pediatric solid tumors, and patients with TRK fusion cancer previously had no targeted treatment options related to their targets, while the OS of NTRK gene fusion tumor patients receiving standard treatments has not been effectively extended[3]. Compounding the issue, patients with NTRK gene fusion tumors often have poor prognoses, with shorter survival times compared to NTRK wild-type tumor patients[4-5]. The emergence of TRK inhibitors is changing this situation. Larotrectinib is a TRK inhibitor that was officially approved for use in China in April 2022 for treating adults and children with locally advanced or metastatic solid tumors positive for NTRK gene fusions, marking the beginning of a new era in precision oncology. In addition to the significant advantages mentioned above compared to SoC, Larotrectinib has also shown satisfactory results in comparisons with immune checkpoint inhibitors. The latest studies released in June this year[6] indicate that in NSCLC patients, compared to Nivolumab (nivo) and Pembrolizumab (pembro), Larotrectinib increased life expectancy (LYs) by 5.87 and 5.91, and quality-adjusted life years (QALYs) by 3.53 and 3.56, respectively. In patients with differentiated thyroid cancer (DTC), compared to Pembrolizumab, Larotrectinib also increased 5.23 LYs and 4.24 QALYs (Table 2). This result indicates that Larotrectinib not only improves patient survival time but also enhances the quality of life of patients.
Table 2 Larotrectinib Compared to Immune Checkpoint Inhibitors
As more long-term survival data accumulates and more patients are included, future research will provide more solid and detailed analyses to validate the efficacy and safety of NTRK inhibitors. Additionally, due to potential biological differences between different tumor types, specific single-tumor studies may provide more reference information for clinical diagnosis and treatment and help optimize patient treatment strategies.
Conclusion
Due to the research difficulties associated with rare targets, high-quality external control studies can effectively utilize existing clinical trial data, real-world data, and even published research data to provide critical evidence support for clinical practice. The re-utilization of clinical trial data not only benefits drug development and optimizes practice, providing more evidence for efficacy and safety comparisons but also inspires new research ideas and generates multidimensional positive effects. In the face of malignant tumors with NTRK gene fusions, Larotrectinib has already proven its efficacy and safety in pan-tumor treatment, and we look forward to its continued efforts in exploring the broad future of TRK inhibitors.
[1]Marcia S. Brose, 2024 ASCO abstract 3105[2]Carsten Bokemeyer, et al. Survival Outcomes of Patients With Tropomyosin Receptor Kinase Fusion-Positive Cancer Receiving Larotrectinib Versus Standard of Care: A Matching-Adjusted Indirect Comparison Using Real-World Data. JCO Precis Oncol 7:e2200436[3]George D. Demetri, et al. Characteristics and outcomes of patients with NTRK fusion-positive (NTRK+) metastatic / locally advanced solid tumours receiving non- TRK inhibitor standard of care, and prognostic value of NTRK fusions in clinical practice. ESMO 2021. 100P[4]Lyudmila Bazhenova, et al. TRK Fusion Cancer: Patient Characteristics and Survival Analysis in the Real‐World Setting. Targeted Oncology. https://doi.org/10.1007/s11523-021-00815-4[5]Irene Santi, et al. Prognostic value of the NTRK fusion biomarker in the Netherlands. ESMO 2021. 105P[6]J Manag Care Spec Pharm. 2024;30(6):581-87
Review Number: MA-LAR-CN-0245-1