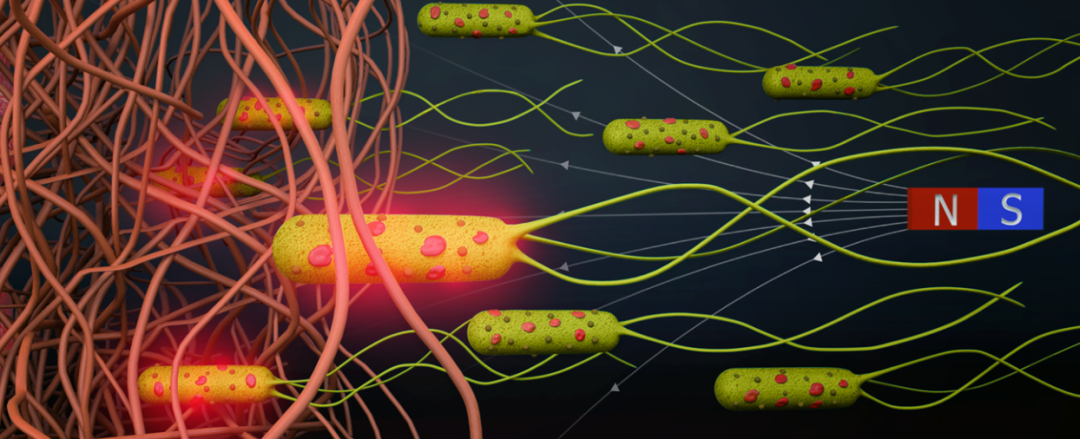
 Written by丨Wang CongEdited by丨Wang DuoyuTypeset by丨Shui ChengwenBacteria are attracted to chemical gradients, such as low oxygen levels or high acidity, which are commonly found near tumor tissues. Injecting bacteria near tumor tissues for cancer treatment is known as bacterial-mediated tumor therapy.Bacteria accumulate in tumor tissues and grow within them, activating the patient’s immune system to combat the tumor.As early as the late 19th century, American surgeon William Coley discovered that bacterial infections could lead to tumor regression and utilized inactivated bacteria to combat malignant tumors, marking one of the earliest forms of cancer immunotherapy.Escherichia coli is a type of multifunctional bacteria that can swim rapidly and navigate through various materials, from liquids to highly viscous tissues, and they possess highly sensitive sensing capabilities. Over the past few decades, scientists have been seeking ways to further enhance the capabilities of E. coli, hoping to equip them with additional components to help them fight cancer cells.Recently, researchers at the Max Planck Institute for Intelligent Systems (MPI-IS) in Germany published a study titled: Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery in the journal Science Advances.This research combines robotics with biology, equipping E. coli with artificial components to construct mobile biohybrid microrobots capable of carrying drug molecules, which can navigate in 3D biological materials under magnetic guidance and deliver anticancer drugs directly to tumors.
Written by丨Wang CongEdited by丨Wang DuoyuTypeset by丨Shui ChengwenBacteria are attracted to chemical gradients, such as low oxygen levels or high acidity, which are commonly found near tumor tissues. Injecting bacteria near tumor tissues for cancer treatment is known as bacterial-mediated tumor therapy.Bacteria accumulate in tumor tissues and grow within them, activating the patient’s immune system to combat the tumor.As early as the late 19th century, American surgeon William Coley discovered that bacterial infections could lead to tumor regression and utilized inactivated bacteria to combat malignant tumors, marking one of the earliest forms of cancer immunotherapy.Escherichia coli is a type of multifunctional bacteria that can swim rapidly and navigate through various materials, from liquids to highly viscous tissues, and they possess highly sensitive sensing capabilities. Over the past few decades, scientists have been seeking ways to further enhance the capabilities of E. coli, hoping to equip them with additional components to help them fight cancer cells.Recently, researchers at the Max Planck Institute for Intelligent Systems (MPI-IS) in Germany published a study titled: Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery in the journal Science Advances.This research combines robotics with biology, equipping E. coli with artificial components to construct mobile biohybrid microrobots capable of carrying drug molecules, which can navigate in 3D biological materials under magnetic guidance and deliver anticancer drugs directly to tumors. The corresponding author of the study, Dr. Metin Sitti, stated that biohybrid microrobots with medical functions could one day more effectively combat cancer, representing a new approach not far from our current cancer treatment methods. The potential of medical microrobots in locating and destroying tumor cells could be significant. This research is also a great example of how basic scientific research benefits society.
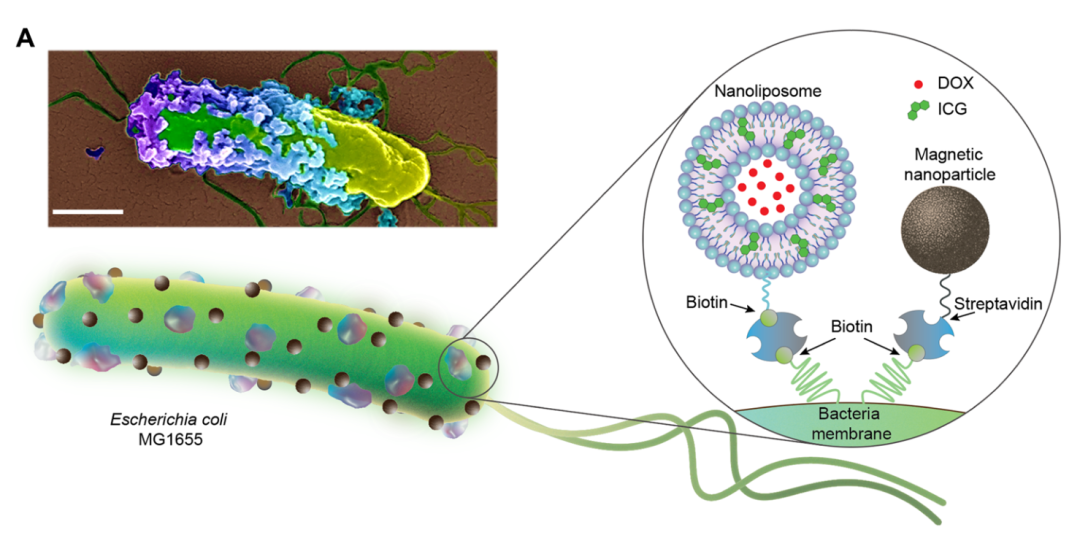
The corresponding author of the study, Dr. Metin Sitti, stated that biohybrid microrobots with medical functions could one day more effectively combat cancer, representing a new approach not far from our current cancer treatment methods. The potential of medical microrobots in locating and destroying tumor cells could be significant. This research is also a great example of how basic scientific research benefits society. Biohybrid microrobots are formed by combining living microorganisms (such as bacteria or algae) with artificial components (such as nanocarriers), creating self-functional micromachines with intrinsic propulsion, sensing, and targeting mechanisms. Among different types of microrobots, bacterial-driven biohybrid microrobots stand out as ideal candidates for medical microrobotic applications.However, in practice, adding artificial components to bacteria is not straightforward, involving complex chemical reactions. Most techniques used to design biohybrid microrobots may adversely affect the bacteria themselves, potentially interfering with bacterial movement or altering their protein expression. Current bacterial biohybrid designs lack high-throughput and easily constructible artificial components, resulting in constructed bacterial hybrid microrobots often performing poorly in propulsion, payload, tissue penetration, and spatiotemporal operation.In this latest study, the research team first attached nanoliposomes to E. coli, which encapsulated the water-soluble chemotherapy drug doxorubicin (DOX). The phospholipid bilayer of these nanoliposomes also embedded indocyanine green (ICG), a medical fluorescent dye that melts under near-infrared light. Thus, this is a photothermally active liposomal plasmid that, upon absorbing near-infrared light (NIR), causes the ICG embedded in the phospholipid bilayer of the nanoliposomes to melt, leading to structural changes in the liposomes and the release of the therapeutic drug contained within.The research team also attached magnetic nanoparticles (iron oxide nanoparticles) to the bacteria, which can control and propel the movement of E. coli under the influence of a magnetic field. Both the nanoliposomes and magnetic nanoparticles are linked to the bacteria using the robust streptavidin-biotin complex.
Biohybrid microrobots are formed by combining living microorganisms (such as bacteria or algae) with artificial components (such as nanocarriers), creating self-functional micromachines with intrinsic propulsion, sensing, and targeting mechanisms. Among different types of microrobots, bacterial-driven biohybrid microrobots stand out as ideal candidates for medical microrobotic applications.However, in practice, adding artificial components to bacteria is not straightforward, involving complex chemical reactions. Most techniques used to design biohybrid microrobots may adversely affect the bacteria themselves, potentially interfering with bacterial movement or altering their protein expression. Current bacterial biohybrid designs lack high-throughput and easily constructible artificial components, resulting in constructed bacterial hybrid microrobots often performing poorly in propulsion, payload, tissue penetration, and spatiotemporal operation.In this latest study, the research team first attached nanoliposomes to E. coli, which encapsulated the water-soluble chemotherapy drug doxorubicin (DOX). The phospholipid bilayer of these nanoliposomes also embedded indocyanine green (ICG), a medical fluorescent dye that melts under near-infrared light. Thus, this is a photothermally active liposomal plasmid that, upon absorbing near-infrared light (NIR), causes the ICG embedded in the phospholipid bilayer of the nanoliposomes to melt, leading to structural changes in the liposomes and the release of the therapeutic drug contained within.The research team also attached magnetic nanoparticles (iron oxide nanoparticles) to the bacteria, which can control and propel the movement of E. coli under the influence of a magnetic field. Both the nanoliposomes and magnetic nanoparticles are linked to the bacteria using the robust streptavidin-biotin complex.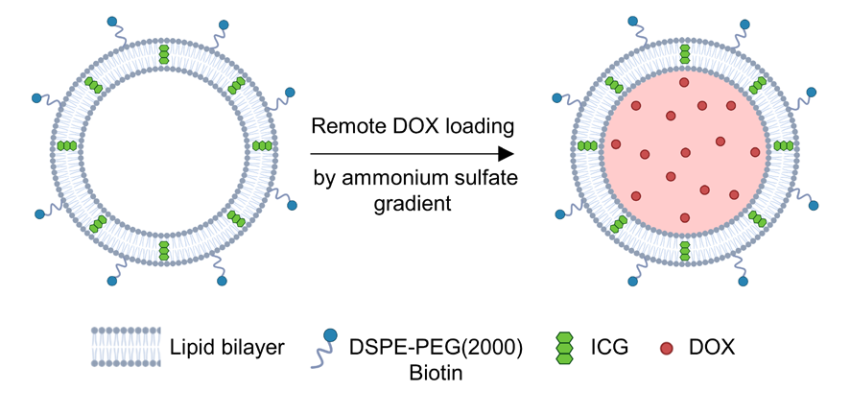
 Next, the research team confirmed in vitro that this bacterial biohybrid microrobot could be precisely navigated by a magnetic field and release chemotherapy drugs towards tumor bodies.Subsequently, the research team further tested in viscous collagen gels (similar to tumor tissues), and the experimental results showed that under the influence of a magnetic field, these bacterial biohybrid microrobots could traverse collagen gels of varying hardness and porosity. This indicates that these bacterial biohybrid microrobots can penetrate and move in confined and porous biological microenvironments under magnetic guidance.
Next, the research team confirmed in vitro that this bacterial biohybrid microrobot could be precisely navigated by a magnetic field and release chemotherapy drugs towards tumor bodies.Subsequently, the research team further tested in viscous collagen gels (similar to tumor tissues), and the experimental results showed that under the influence of a magnetic field, these bacterial biohybrid microrobots could traverse collagen gels of varying hardness and porosity. This indicates that these bacterial biohybrid microrobots can penetrate and move in confined and porous biological microenvironments under magnetic guidance. Once these microrobots gather around the tumor, near-infrared light (NIR) is used to trigger the melting process of the nanoliposomes and release the chemotherapy drugs they carry. The acidic environment near the tumor with low pH also causes the nanoliposomes to rupture, thus automatically releasing the drugs near the tumor.
Once these microrobots gather around the tumor, near-infrared light (NIR) is used to trigger the melting process of the nanoliposomes and release the chemotherapy drugs they carry. The acidic environment near the tumor with low pH also causes the nanoliposomes to rupture, thus automatically releasing the drugs near the tumor.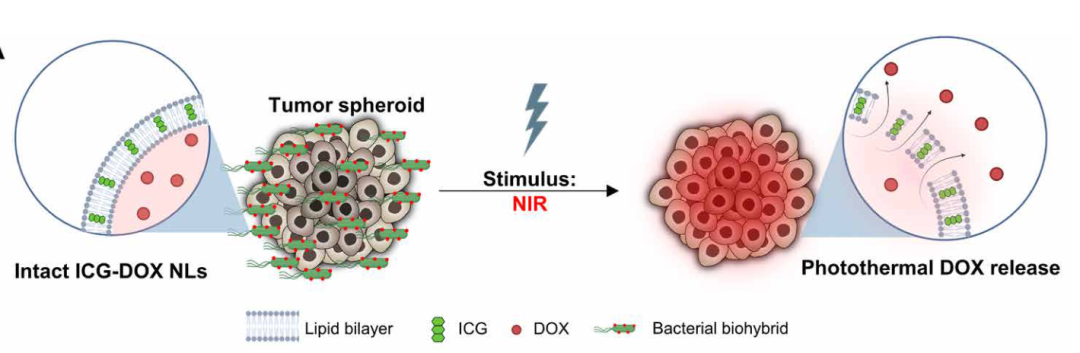 Imagine injecting these bacteria-based microrobots into cancer patients, where they can navigate to the tumor under magnetic guidance. Once enough microrobots surround the tumor, near-infrared light can be activated to trigger the release of chemotherapy drugs. This not only awakens the immune system but also helps destroy the tumor by releasing chemotherapy drugs. This delivery method is minimally invasive, painless, and has minimal toxicity, with the drugs acting directly on the tumor, avoiding harm to other parts of the body.Overall, the bacterial biohybrid microrobots developed in this latest study outperform previously reported E. coli-based microrobots, retaining the motility of E. coli itself while demonstrating the ability to navigate and colonize tumors under magnetic guidance, and then release the loaded anticancer drugs as needed.In summary, the bacterial biohybrid design presented here provides a systematic, high-throughput platform for multifunctional medical microrobots that can overcome biological barriers and perform stimuli-responsive active drug release.Paper link: https://www.science.org/doi/10.1126/sciadv.abo6163
Imagine injecting these bacteria-based microrobots into cancer patients, where they can navigate to the tumor under magnetic guidance. Once enough microrobots surround the tumor, near-infrared light can be activated to trigger the release of chemotherapy drugs. This not only awakens the immune system but also helps destroy the tumor by releasing chemotherapy drugs. This delivery method is minimally invasive, painless, and has minimal toxicity, with the drugs acting directly on the tumor, avoiding harm to other parts of the body.Overall, the bacterial biohybrid microrobots developed in this latest study outperform previously reported E. coli-based microrobots, retaining the motility of E. coli itself while demonstrating the ability to navigate and colonize tumors under magnetic guidance, and then release the loaded anticancer drugs as needed.In summary, the bacterial biohybrid design presented here provides a systematic, high-throughput platform for multifunctional medical microrobots that can overcome biological barriers and perform stimuli-responsive active drug release.Paper link: https://www.science.org/doi/10.1126/sciadv.abo6163



 Open for ReprintFeel free to share in your circle of friends and WeChat groups WeChat GroupTo promote the dissemination and exchange of cutting-edge research, we have established several professional discussion groups. Long press the QR code below to add the editor’s WeChat to join the group. Due to the high number of applicants, please note your: School/Major/Name when adding WeChat. If you are a PI/Professor, please also indicate.
Open for ReprintFeel free to share in your circle of friends and WeChat groups WeChat GroupTo promote the dissemination and exchange of cutting-edge research, we have established several professional discussion groups. Long press the QR code below to add the editor’s WeChat to join the group. Due to the high number of applicants, please note your: School/Major/Name when adding WeChat. If you are a PI/Professor, please also indicate.
Click to see your taste