As early as 1913, during the 17th International Medical Congress, Professor Paul Ehrlich proposed the concept of the “magic bullet”, using monoclonal antibodies to specifically deliver cytotoxic drugs to tumor cells, thereby achieving the goal of killing tumor cells.
With the development of monoclonal antibody production technology and the exploration of antibody conjugates, antibody-drug conjugates (ADC) have been realized and rapidly applied in clinical settings.
Figure: Professor Paul Ehrlich
There are several key points in the development of ADC drugs:
Selection of Target Antigens: The target antigen needs to be specifically expressed on the cell membrane of tumor cells, which facilitates the binding of monoclonal antibodies; moreover, if the expression of the target antigen is related to cancer prognosis, it is even more beneficial for ADC to exert its effects.
Requirements for Linkers: The linker needs to have appropriate stability. It should avoid the premature release of cytotoxic drugs in peripheral blood, causing systemic toxicity, while allowing for efficient release once internalized into tumor cells, achieving the goal of killing tumors.
Selection of Cytotoxic Drugs: Needs to have appropriate hydrophobicity. Hydrophobic linkers combined with hydrophobic cytotoxic molecules can promote the aggregation of ADC molecules, which may cause immune reactions during blood circulation.
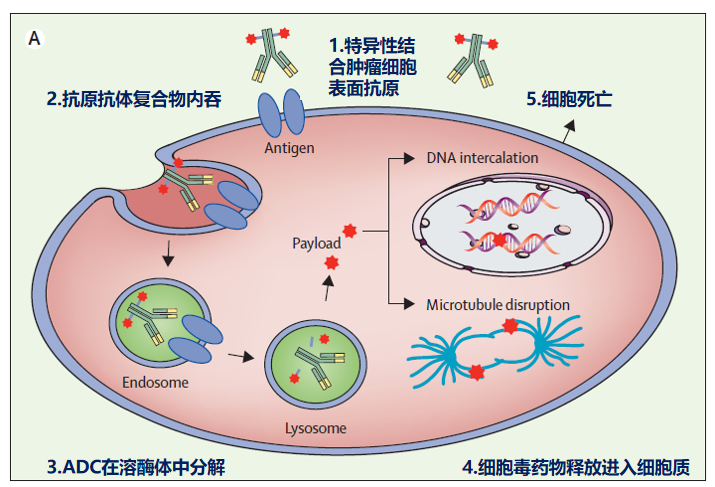
Figure: Mechanism of ADC drugs
Trastuzumab emtansine (T-DM1) is the first ADC drug used for the treatment of breast cancer. On January 18, 2023, it officially entered the National Basic Medical Insurance, Work Injury Insurance, and Maternity Insurance Drug Catalog (2022 edition) for all indications, and the national medical insurance payment standards were simultaneously determined.
ADC drugs have become a class of drugs that can be commonly used by HER2-positive breast cancer patients in China. Taking this opportunity, let’s review some common ADC drugs in the field of breast cancer:
Trastuzumab Emtansine (T-DM1) …
T-DM1 is an ADC drug targeting the HER2 protein, linked to the microtubule inhibitor DM1 via an irreversible linker based on trastuzumab, thereby killing tumor cells.
EMILIA Study [1] is a prospective, randomized controlled, Phase III clinical study that enrolled 991 HER2-positive advanced breast cancer patients, with the experimental group receiving T-DM1 monotherapy and the control group receiving lapatinib plus capecitabine. The results showed that the median PFS in the experimental and control groups was 9.6 months and 6.4 months, respectively, HR 0.65; 95% CI, 0.55-0.77; P<0.001. The results made T-DM1 monotherapy one of the standard treatment options for HER2-positive advanced second-line therapy.
KATHERINE Study [2] is a prospective, randomized controlled, Phase III clinical study that enrolled 1486 HER2-positive early breast cancer patients who did not achieve pCR after neoadjuvant therapy. The experimental group received T-DM1 monotherapy for 14 cycles, while the control group received trastuzumab monotherapy for 14 cycles. The results showed that the 3-year iDFS for the two groups was 88.3% and 77.0%, respectively, HR 0.50; 95% CI, 0.39-0.64; P<0.001.
The study results proved that for HER2-positive early high-risk breast cancer patients who received neoadjuvant therapy and did not achieve pCR, T-DM1 monotherapy was superior to the then standard treatment of trastuzumab monotherapy. The KATHERINE study explored a new treatment model for early HER2-positive breast cancer patients, allowing the identification of high-risk patients who require intensified adjuvant therapy, making T-DM1 one of the standard treatment options for this group.
Like T-DM1, T-DXd is also an anti-HER2 ADC drug developed based on trastuzumab. However, unlike T-DM1, T-DXd has a unique linker and cytotoxic drug payload DXd. DXd is a topoisomerase inhibitor that can effectively kill tumor cells and is not commonly found in the treatment history of advanced breast cancer patients.
Additionally, T-DXd has a unique cleavable linker with a four-peptide (GGFG) that allows for a higher drug payload while exerting a bystander effect. Thanks to the single-arm clinical study DESTINY-Breast01, T-DXd was granted FDA approval ahead of schedule.
DESTINY-Breast03 Study [3] is a prospective, randomized controlled, Phase III clinical study that enrolled 524 HER2-positive advanced breast cancer patients, with the experimental group receiving T-DXd and the control group receiving T-DM1.
The results showed that the median PFS in the experimental and control groups was 28.8 months and 6.8 months, respectively, HR 0.33; 95% CI, 0.47-0.87; P<0.000001. The OS also showed significant differences between the two groups, HR 0.64; 95% CI, 0.47-0.87; P = 0.0037. The results made T-DXd one of the standard treatment options for HER2-positive advanced second-line therapy.
DESTINY-Breast04 Study [4]is a prospective, randomized controlled, Phase III clinical study that enrolled 557 HER2-low expressing advanced second and third-line breast cancer patients, with the experimental group receiving T-DXd and the control group receiving a physician’s choice of chemotherapy.
The results showed that the median PFS in the experimental and control groups was 9.9 months and 5.1 months, respectively, HR 0.50; 95% CI, 0.40-0.63; P < 0.001. The median OS for the experimental and control groups was 23.4 months and 16.8 months, respectively, HR 0.64; 95% CI, 0.49-0.84; P = 0.001. This study successfully demonstrated the necessity of using anti-HER2 treatment in HER2-low expressing populations and quickly received FDA approval, being the only FDA-approved anti-HER2 drug for HER2-low expressing populations to date.
Sacituzumab Govitecan (SG) …
SG is an ADC drug targeting the Trop-2 protein, loading the topoisomerase inhibitor SN-38 based on the Trop-2 targeting monoclonal antibody.
ASCENT Study [5] is a prospective, randomized controlled, Phase III clinical study that enrolled 468 patients with advanced triple-negative breast cancer, with the experimental group receiving SG and the control group receiving a physician’s choice of chemotherapy. The results showed that the median PFS in the experimental and control groups was 5.6 months and 1.7 months, respectively, HR 0.41; 95% CI, 0.32-0.52; P<0.001.
The median OS for the experimental and control groups was 12.1 months and 6.7 months, respectively, HR 0.48; 95% CI, 0.38-0.59; P<0.001. Interestingly, further analysis found that patients could benefit from SG treatment regardless of whether they overexpress Trop-2. The results made SG one of the standard treatment options for advanced triple-negative breast cancer.
TROPiCS-02 Study [6] is a prospective, randomized controlled, Phase III clinical study that enrolled 543 patients with HR-positive HER2-negative advanced breast cancer, with the experimental group receiving SG and the control group receiving a physician’s choice of chemotherapy.
The results showed that the median PFS in the experimental and control groups was 5.4 months and 4.0 months, respectively, HR 0.66; 95% CI, 0.53-0.83; P = 0.0003. The median OS for the experimental and control groups was 14.4 months and 11.2 months, respectively, HR 0.79; 95% CI, 0.65-0.96; P = 0.02. The results made SG one of the standard treatment options for HR-positive advanced second-line therapy and received FDA approval.
The aforementioned approved ADC drugs continue to expand their indications, such as T-DXd, which, due to its successful clinical study results, has corresponding studies ongoing in the advanced first-line and (neo)adjuvant treatment stages for HER2-low expressing and HER2-positive breast cancer, with expectations for more clinical results in the future.
In addition to the above FDA-approved ADC drugs, there are currently multiple single-target ADC drugs under development, such as ARX788, RC-48, SHR1811 targeting HER2, and SKB264, DS1062, and SHR-A1921 targeting Trop-2. Furthermore, there are multi-target ADC drugs worth looking forward to, such as ADC drugs targeting Claudin-18.2 and LIV-1.
Looking back to 1913, when Professor Paul Ehrlich proposed the concept of the “magic bullet”, it is finally being widely applied in clinical practice today. We can fully believe that more new therapeutic drugs will join the treatment of breast cancer in the future, and with the continuous expansion and updating of new drug development technologies such as gene therapy, tumor vaccines, and protein degradation, breast cancer patients will have more and better treatment options.

Author: Dan Ke Long; Review: Lu Yimin
Typesetting: Lin Shuy; Cover Image: Zhanzku Hailuo PLUS
Submission: [email protected]

References
[1] Verma S, Miles D, Gianni L, et al; EMILIA Study Group. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012 Nov 8;367(19):1783-91.
[2] von Minckwitz G, Huang CS, et al; KATHERINE Investigators. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019 Feb 14;380(7):617-628.
[3] Sara A. Hurvitz, a Roberto Hegg, et al; Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results of the randomized, phase 3 study DESTINY-Breast03; 2022 SABCS, GS2-02
[4] Modi S, Jacot W, et al; DESTINY-Breast04 Trial Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med. 2022 Jul 7;387(1):9-20.
[5] Bardia A, Hurvitz SA, et al; ASCENT Clinical Trial Investigators. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2021 Apr 22;384(16):1529-1541.
[6] Rugo HS, Bardia A, et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J Clin Oncol. 2022 Oct 10;40(29):3365-3376.