*For medical professionals only

On October 7, the official website of the National Health Commission announced that 2′-fucosyllactose (2′-FL) and lacto-N-neotetraose (LNnT), two human milk oligosaccharides (HMOs), have been officially approved for use as food nutritional fortifiers in infant formulas, follow-on formulas for older infants and toddlers, and special medical purpose infant formulas. HMOs are the third largest component in breast milk, following fat and lactose, and play a role in promoting the immune development of infants [1]. They are considered one of the most significant research achievements in infant nutrition in recent years. With increasing research on HMOs, their role in managing cow’s milk protein allergy (CMPA) is gradually being recognized. Multiple studies have confirmed that adding HMOs to special medical formulas can simulate the benefits of breast milk and promote immune maturation in CMPA children while ensuring safety and low allergenicity. The approval of HMOs also signifies a move towards more breast milk-like nutritional management for children with cow’s milk protein allergy.
1. Health Benefits of Human Milk Oligosaccharides for Infants

Some studies have found that breastfed infants have a lower incidence of infectious diseases caused by viruses, bacteria, and parasites, which may be related to HMOs [2]. HMOs are unique bioactive components in human milk, composed of five monosaccharide structural units, with complex and diverse structures, and over 200 types have been identified. 2′-FL and LNnT are the two most abundant HMOs in breast milk [3].
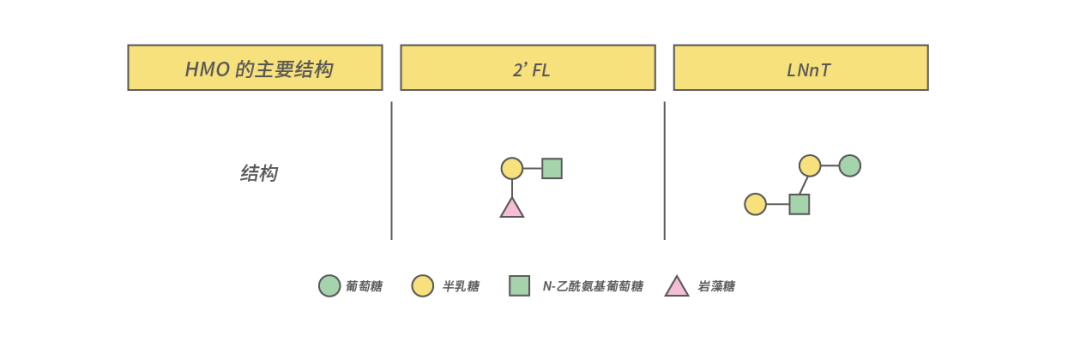
The content of HMOs is higher in the early stages of breastfeeding, which may indicate their important protective role during the period when infants have the weakest immunity [1]. Recent studies have also confirmed that the complex and diverse HMOs provide various health benefits for infants, such as selectively promoting beneficial bacteria colonization, aiding intestinal immune development, reducing pathogen infections, and directly promoting immune maturation in infants.
01
Benefit 1: Selectively Promoting Beneficial Bacteria Colonization
The composition of the gut microbiota plays a crucial role in promoting immune system development and maintaining overall health. Bifidobacteria are the dominant strains in breastfed infants. Studies have compared the immune status of infants with rich bifidobacteria in their gut to those lacking bifidobacteria [4]. The results showed that the group of infants lacking bifidobacteria was more prone to systemic or intestinal inflammation. Some studies have also found that the gut microbiota of CMPA children differs from that of healthy infants, showing a dysbiotic state with increased anaerobes and lactobacilli, and decreased bifidobacteria [5].

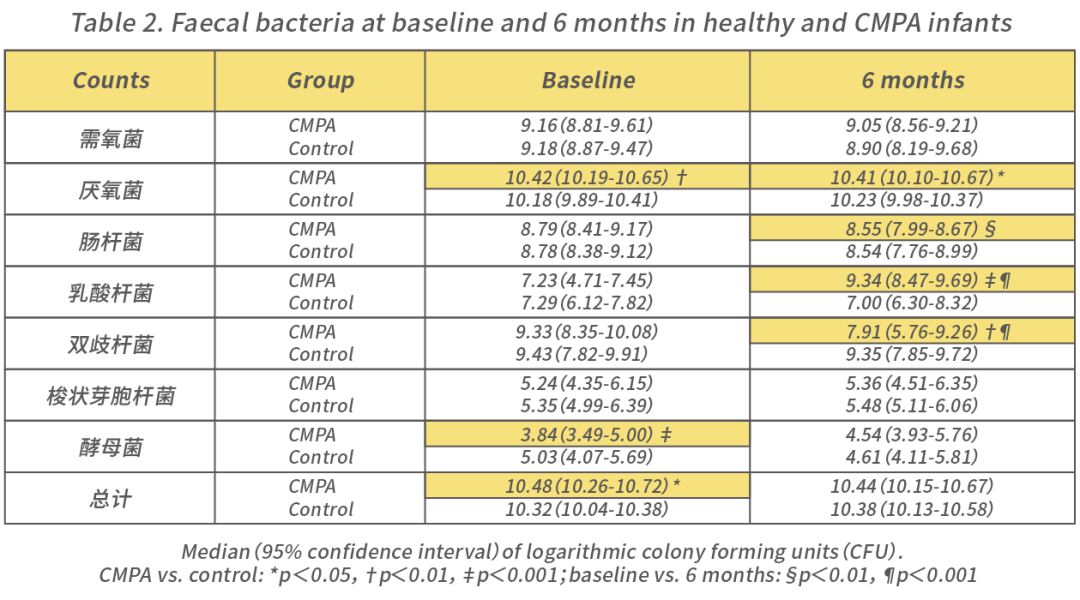
Note [1]: Compared to healthy infants, CMPA infants at 6 months have higher numbers of anaerobes and lactobacilli in their feces, a higher proportion of lactobacilli, and lower numbers and proportions of bifidobacteria.
HMOs are important nutritional substrates for bifidobacteria, selectively promoting their proliferation and inhibiting the growth of harmful bacteria by competing for relatively limited nutritional supplies, thus maintaining a healthy gut microbiota in infants. A study in 2020 confirmed that at 3 months, the gut microbiota composition of infants in the HMO group was closer to that of breastfed infants, with a higher abundance of bifidobacteria and lower levels of Escherichia coli and Enterococcus [6].
02
Benefit 2: Strengthening the Intestinal Immune Barrier
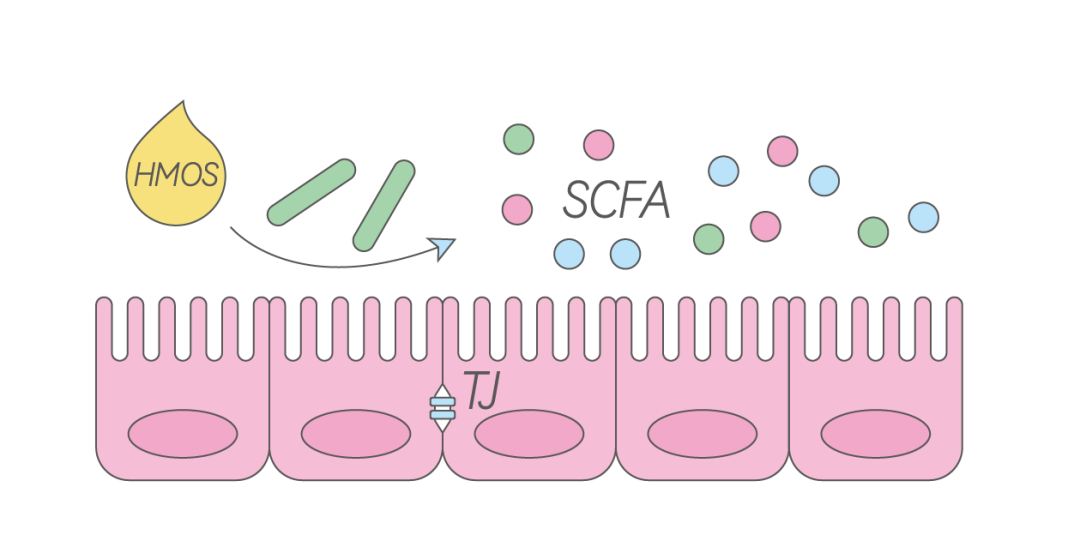
The intestinal barrier is the first line of defense in innate immunity. If the intestinal barrier develops poorly, leading to compromised integrity, it can trigger various immune responses, including gastrointestinal infections, inflammatory bowel disease (IBD), and allergies [7]. Studies have shown that HMOs such as LNnT and 2′-FL can promote the expression of tight junction proteins and increase cell differentiation along the crypt-villus axis, as well as reduce epithelial barrier permeability [8]. Other studies have found that HMOs can directly interact with intestinal epithelial cells and regulate their glycan expression, thereby enhancing cell barrier function [9,10]. This effect is structurally specific, as other types of oligosaccharides such as fructooligosaccharides (FOS) and galactooligosaccharides (GOS) have not shown this effect.
Additionally, during the metabolism of HMOs by bifidobacteria, various short-chain fatty acids are produced, which are an important energy source for intestinal barrier cells and can improve intestinal barrier function [1].
03
Benefit 3: Defending Against Pathogen Infections
Many pathogens need to adhere to mucosal surfaces to colonize or invade the host and cause disease. HMOs directly reduce microbial infections by acting as anti-adhesives. Pathogen adhesion is usually caused by lectin-glycan interactions, and some HMO structures resemble the glycans on the surface of mucosal cells, allowing them to “mimic” the surface glycans of epithelial cells and serve as soluble decoy receptors, preventing pathogens from binding to mucosal cell surfaces and reducing the risk of infection [1,2,11].
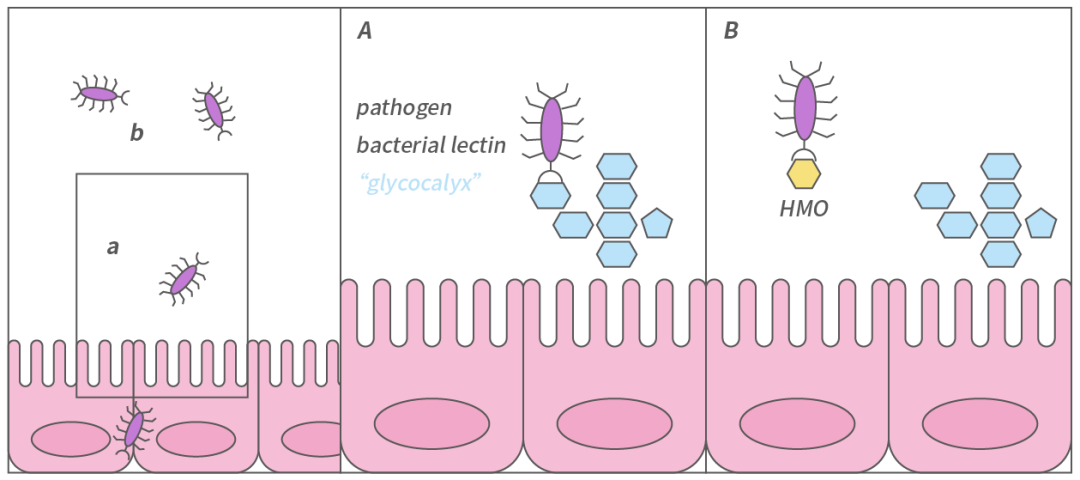
Moreover, when HMOs are fermented in the intestine, they can create an acidic environment that is unfavorable for the growth of most pathogenic microorganisms, thereby reducing the risk of infection [12]. A study in 2017 confirmed that infants in the HMO intervention group reported fewer cases of bronchitis and lower respiratory tract infections, with less use of antipyretics and antibiotics [13].
04
Benefit 4: Directly Promoting Immune Maturation
HMOs not only function in the infant’s gut but also directly promote immune maturation. HMOs are believed to modulate the interactions between immune cells through receptors expressed on the surface of immune cells to balance inflammatory responses. They are also thought to regulate the expression of inflammatory markers important for cell transport and help balance pro-inflammatory and anti-inflammatory cytokines in neonatal immune responses [10,14]. For example, one study showed that HMOs isolated from colostrum reduced the expression of pro-inflammatory cytokines and shifted the balance of Th1/Th2 cytokines to a more mature state [14]. A study in 2016 confirmed that compared to formula-fed infants supplemented with GOS, breastfed infants and those fed formula supplemented with 2′-FL had significantly lower plasma concentrations of inflammatory cytokines [15]. These studies indicate that HMOs can act systemically, potentially directly controlling immune responses by altering the quantity of immune cells and the release of cytokines in infants.
2. Applications of Human Milk Oligosaccharides in Managing Cow’s Milk Protein Allergy

HMOs also play an important role in the management of CMPA. The occurrence of CMPA is related to abnormalities in immune system and gut development, and its management has primarily focused on strict avoidance of cow’s milk protein. However, with increasing research on HMOs, multiple clinical studies have shown that using special medical formulas with added HMOs for CMPA children not only effectively alleviates allergic symptoms but also supports normal growth and development while promoting immune development in CMPA children. These studies suggest that, in addition to passive avoidance, CMPA management can also actively accelerate the process of establishing immune tolerance in CMPA children with the help of HMOs.
For example, the 2022 CINNAMON study showed [16] that using a deeply hydrolyzed formula supplemented with 2′-FL and LNnT for CMPA children up to 12 months helps regulate their gut microbiota and slows down the premature transition to an adult-type microbiota.

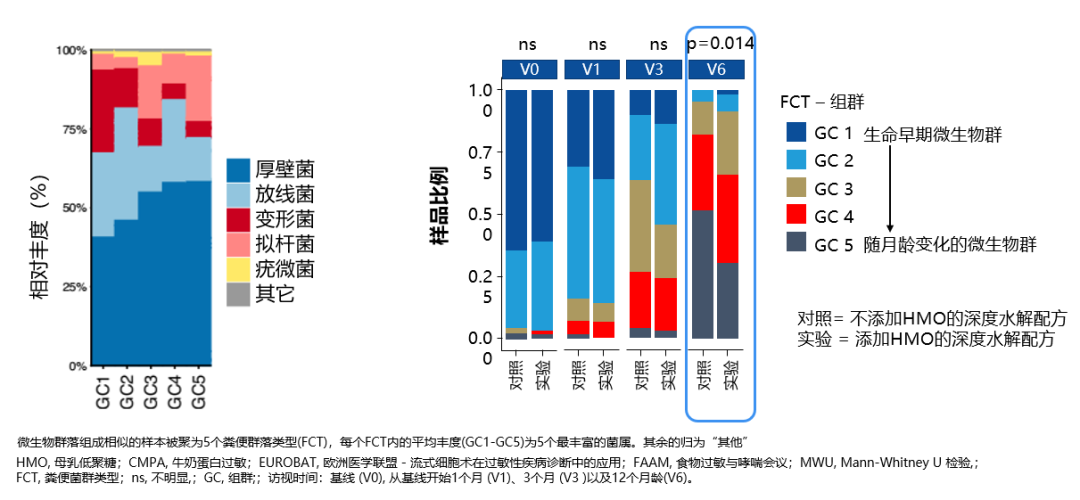
Note [16]: Using eHF containing HMOs can promote the enrichment of bifidobacteria and reduce the abundance of phylum Proteobacteria (such as Escherichia coli), helping to reverse gut dysbiosis. This effect is more pronounced for CMPA infants who start feeding with eHF containing HMOs before 3 months of age.
Additionally, CMPA infants experience fewer respiratory, ear, and gastrointestinal infections, with reduced use of antibiotics and antipyretics.

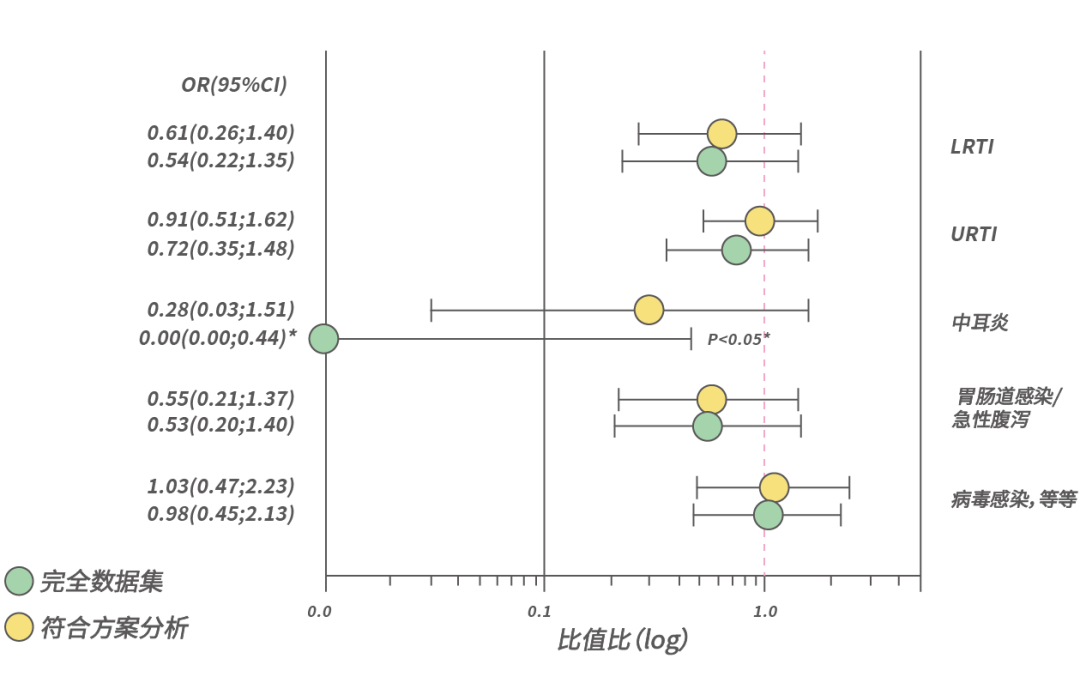
Note [17]: The incidence of infectious diseases in CMPA children decreased after using a special medical formula containing two HMOs.
LRTI – Lower Respiratory Tract Infection; URTI – Upper Respiratory Tract Infection
Another study, the PLATYPUS study, showed [18] that feeding moderate to severe CMPA children with an amino acid formula supplemented with 2′-FL and LNnT resulted in good tolerance. During the 4-month study period, all measured parameters developed normally and showed an upward trend.

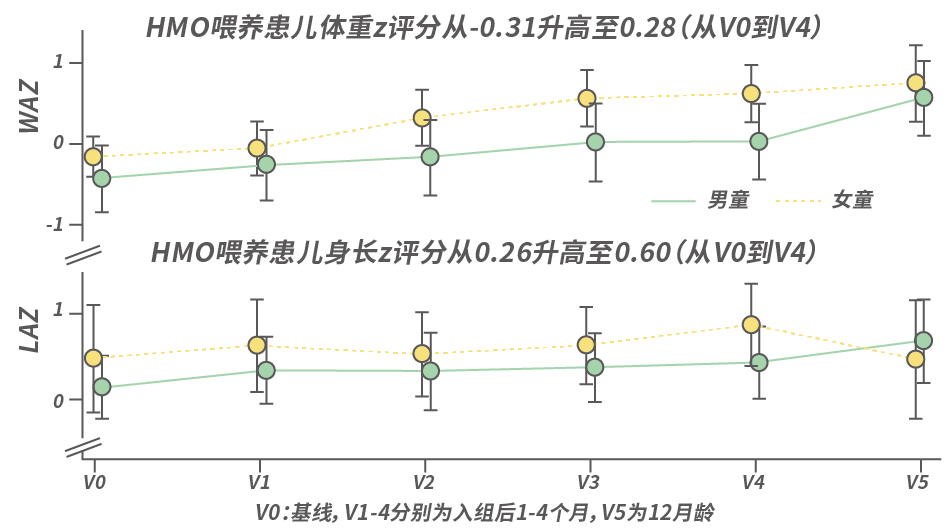
Note [18]: After using an amino acid formula containing two HMOs, the gut microbiota of CMPA children showed positive regulation.
Breast milk is the best food for infants, and both infant formulas and special medical purpose infant formulas have been striving to incorporate breast milk components over the years. With the official approval of these two HMOs, it means that the nutritional management of CMPA children who are artificially fed will further approach breast milk on a safe and effective basis, helping more CMPA children establish immune tolerance early and promote normal growth.
Conclusion
-
The National Health Commission has approved 2′-fucosyllactose (2′-FL) and lacto-N-neotetraose (LNnT) as food nutritional fortifiers to be added to infant formulas, follow-on formulas for older infants and toddlers, and special medical purpose infant formulas.
-
Human milk oligosaccharides are unique bioactive components in human milk that provide various health benefits for infants, such as selectively promoting beneficial bacteria colonization, aiding intestinal immune development, reducing pathogen infections, and directly promoting immune maturation.
-
Special medical formulas containing these two human milk oligosaccharides also play an important role in managing cow’s milk protein allergy. They can reduce the infection rate in CMPA children while ensuring safety, low allergenicity, and promoting normal growth.
References:
[1].Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012 Sep;22(9):1147-62.
[2].Sekerel BE, Bingol G, et al. An Expert Panel Statement on the Beneficial Effects of Human Milk Oligosaccharides (HMOs) in Early Life and Potential Utility of HMO-Supplemented Infant Formula in Cow’s Milk Protein Allergy. J Asthma Allergy. 2021 Sep 24;14:1147-1164.
[3].Dinleyici M, et al. Functional effects of human milk oligosaccharides (HMOs)[J]. Gut microbes, 2023, 15(1): 2186115.
[4].Henrick B M, Rodriguez L, Lakshmikanth T, et al. Bifidobacteria-mediated immune system imprinting early in life[J]. Cell, 2021, 184(15): 3884-3898. e11.
[5].Thompson-Chagoyan OC, Vieites JM, Maldonado J, Edwards C, Gil A. Changes in faecal microbiota of infants with cow’s milk protein allergy–a Spanish prospective case-control 6-month follow-up study. Pediatr Allergy Immunol. 2010 Mar;21(2 Pt 2):e394-400.
[6].Berger B, et al. Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk To Require Antibiotics. mBio. 2020 Mar 17;11(2):e03196-19.
[7].Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease [J]. Expert Review of Gastroenterology & Hepatology, 2017, 11(9):821-34.
[8].Holscher HD, Davis SR, Tappenden KA. Human milk oligosaccharides influence maturation of human intestinal Caco-2Bbe and HT-29 cell lines. J Nutr. 2014 May;144(5):586-91.
[9].Angeloni S, et al. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology. 2005 Jan;15(1):31-41.
[10].Triantis V, Bode L, van Neerven RJJ. Immunological Effects of Human Milk Oligosaccharides. Front Pediatr. 2018 Jul 2;6:190.
[11].Donovan SM, Comstock SS. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann Nutr Metab. 2016;69 Suppl 2(Suppl 2):42-51.
[12].Jiang S, et al. The role of human milk oligosaccharides in infant growth and development and its influencing factors[J]. Chinese Journal of Maternal and Child Health Research, 2023, 34(3):8.
[13].PUCCIO G, et al. Effects of Infant Formula With Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial [J]. J Pediatr Gastroenterol Nutr, 2017, 64(4): 624-31.
[14].He Y, Liu S, Leone S, Newburg DS. Human colostrum oligosaccharides modulate major immunologic pathways of immature human intestine. Mucosal Immunol. 2014 Nov;7(6):1326-39.
[15].Goehring KC, et al. Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. The Journal of nutrition. 2016 Dec 1;146(12):2559-66.
[16].Boulangé C L, et al. An Extensively Hydrolyzed Formula Supplemented with Two Human Milk Oligosaccharides Modifies the Fecal Microbiome and Metabolome in Infants with Cow’s Milk Protein Allergy[J]. International Journal of Molecular Sciences, 2023, 24(14): 11422.
[17].Vandenplas Y, et al. Effects of an Extensively Hydrolyzed Formula Supplemented with Two Human Milk Oligosaccharides on Growth, Tolerability, Safety and Infection Risk in Infants with Cow’s Milk Protein Allergy: A Randomized, Multi-Center Trial[J]. Nutrients. 2022; 14(3):530.
[18].Gold M S, et al. Effects of an Amino Acid-Based Formula Supplemented with Two Human Milk Oligosaccharides on Growth, Tolerability, Safety, and Gut Microbiome in Infants with Cow’s Milk Protein Allergy[J]. Nutrients, 2022, 14(11): 2297.
*The “Medical Community” strives for the content published to be professional and reliable, but does not guarantee the accuracy of the content; relevant parties should verify separately when adopting or using it as a basis for decision-making.

↓↓↓ Click “Read Original” to learn more clinical skills