Liver failure is a severe destruction of hepatocytes triggered by various pathological factors, leading to significant liver dysfunction. In clinical practice, it is often characterized by ascites, jaundice, hepatorenal syndrome, and infections, commonly associated with systemic inflammatory response syndrome. Its onset is rapid, the progression is swift, and it has many complications, making it extremely dangerous and even life-threatening for patients. Acute pancreatitis is an inflammatory response formed due to the activation of pancreatic enzymes within the pancreas caused by various stimuli, which can even lead to organ dysfunction and acute abdomen.The incidence of acute pancreatitis complicating liver failure is approximately 40%, and acute pancreatitis can further exacerbate liver damage, leading to worsened liver function, poor prognosis, and high mortality rates.The clinical symptoms of acute pancreatitis are extremely similar to the clinical manifestations of liver failure and may be overlooked, resulting in missed diagnoses. This article elaborates on the mechanisms and preventive measures for acute pancreatitis complicating liver failure, providing a reference for the prevention and treatment of acute pancreatitis in patients with clinical liver failure.

1Inflammatory Response
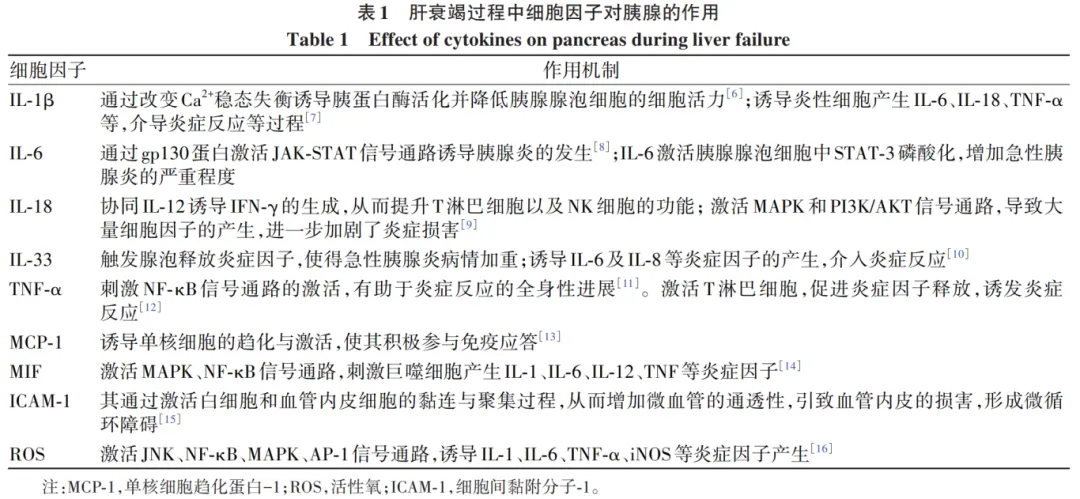
During the evolution of liver failure, the inflammatory response triggered by M1 macrophages releases several cytokines, such as IL-1β, IL-6, IL-10, IL-18, IL-33, TNF-α, and macrophage migration inhibitory factor (MIF), leading to inflammation in the pancreas. Research related to liver failure diseases has shown that during its progression, the expression level of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in hepatocyte nuclei significantly increases. The activation of NF-κB is a key early event in the inflammatory progression of acute pancreatitis; during liver failure, various cytokines’ expression levels in the body significantly increase, inducing the progression of acute pancreatitis. The impact of cytokines on the pancreas during liver failure is shown in Table 1.
Many cytokines released during the progression of liver failure constitute the material basis for triggering acute pancreatitis. After stimulation by cholecystokinin-8 or carbachol, protein kinase C undergoes phosphorylation and activation, subsequently activating the NF-κB pathway and releasing inflammatory factors, leading to the occurrence of the inflammatory response. The JAK pathway for releasing inflammatory factors is similar. The inflammatory factor TNF-α released during liver failure can activate T lymphocytes, stimulating the activation of the NF-κB signaling pathway, inducing the systemic progression of the inflammatory response. TNF-α can also promote the production of NO, reactive oxygen species (ROS), and other inflammatory cytokines by increasing the levels of intercellular ICAM and vascular cell adhesion molecules. P21-activated kinase 1 can promote the production of inflammatory factors TNF-α, IL-6, IL-1β by activating the NF-κB and p38 pathways, mediating the inflammatory response. Toll-like receptor 4 (TLR4) has the ability to recognize pancreatic secreted enzymes and various cytokine signals, further releasing the first signal that triggers the inflammatory response, leading to a cascade of inflammation. This process activates and increases the protein level of TNF receptor-associated factor 6, enhancing downstream signal transmission and causing the NF-κB subunit to translocate from the cytoplasm to the nucleus. In the nucleus, NF-κB transcription factors stimulate the expression of inflammatory cytokine genes. In hepatocytes, high mobility group box 1 (HMGB1) is the last late inflammatory mediator to appear and has the longest duration during systemic inflammation; it can activate MAPK and NF-κB signaling pathways by binding with TLR4, promoting the release of inflammatory factor MIF, further exacerbating the inflammatory response. The NLRP-3 inflammasome can mediate the activation of Caspase-1, which activates inflammatory factors IL-1β, IL-18, exacerbating the inflammatory response during acute pancreatitis, and even further inducing the aggravation of liver failure (Figure 1).
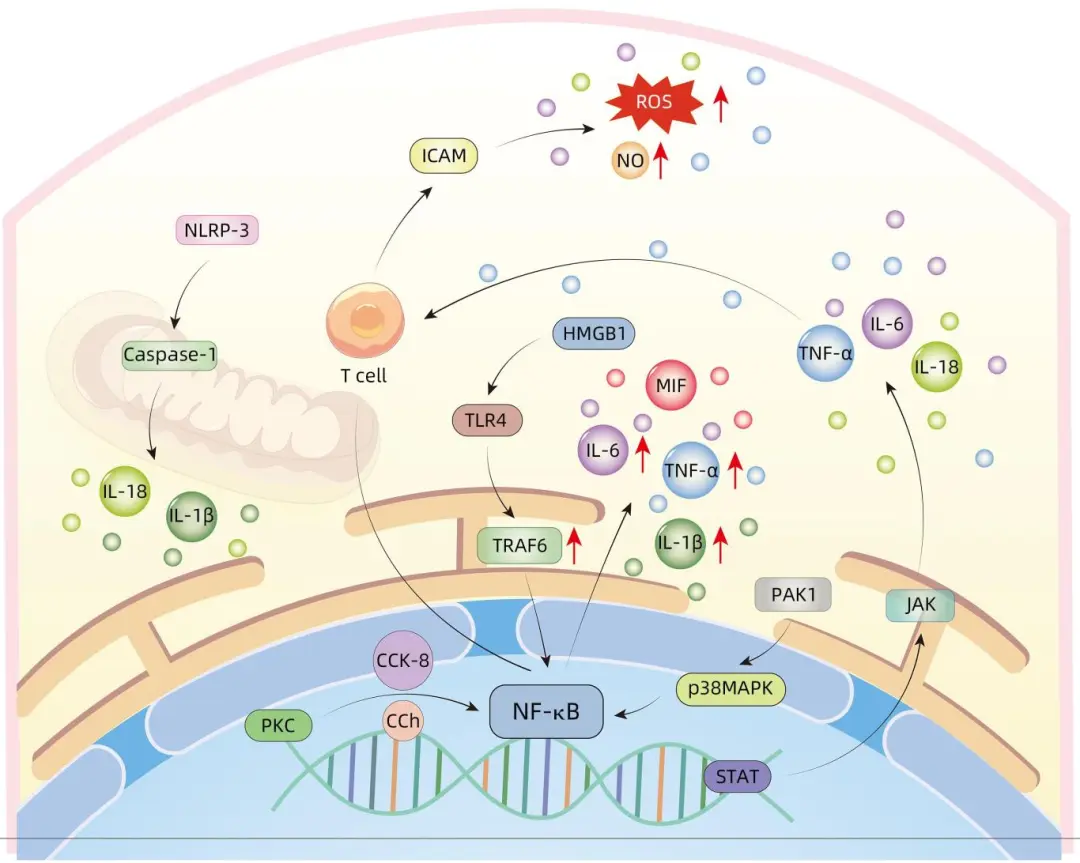
Figure 1: Mechanism of Inflammatory Response in Acute Pancreatitis Complicating Liver Failure

2Duodenal Papilla Dysfunction
Duodenal papilla dysfunction includes two major categories: duodenal sphincter stenosis and motility disorders. Duodenal sphincter stenosis includes partial or complete stenosis of the duodenal sphincter caused by inflammation and secondary fibrosis. Motility disorders occur due to changes in normal physiological movements, leading to the inability of bile or pancreatic juice to flow into the duodenum. Dysfunction of the duodenal papilla can not only induce inflammation but also lead to dysbiosis of the intestinal and biliary microbiota, resulting in an increase in pathogenic microbial populations.During the progression of liver failure, the liver’s tissue structure is destroyed, bile flows through the common bile duct into the duodenum via the duodenal sphincter, and spasm of the duodenal sphincter causes bile to reflux into the pancreatic duct.This leads to an increase in pressure in the bile and pancreatic ducts, activation of trypsinogen, and auto-digestion of the pancreas, inducing acute pancreatitis.The activation of trypsinogen within the acini plays a critical role in pancreatitis; in transgenic mouse models where trypsinogen is specifically activated, increased trypsin activity leads to tissue edema, serum amylase, inflammatory cell infiltration, and acinar cell apoptosis, inducing acute pancreatitis.At the same time, dysfunction of the duodenal papilla leads to mitochondrial dysfunction in pancreatic duct cells, resulting in premature activation of trypsinogen in the pancreatic duct, triggering acute pancreatitis.

3Intestinal Dysbiosis
The intestinal microbiota refers to various microorganisms residing in the intestines, whose roles include intestinal protection, optimizing metabolism, regulating the immune system, combating inflammation, and anti-tumor functions. In liver failure, the balance of the microbiota is disrupted. Moreover, the degree of dysbiosis correlates positively with the extent of hepatocyte injury. During the progressive deterioration of liver function, intestinal microbiota imbalance occurs, with excessive proliferation of microbiota, microbial translocation, and disruption of the intestinal mucosal barrier. These adverse conditions further accelerate the occurrence and development of pancreatitis. A decrease in intestinal lactobacilli and dysfunction of Paneth cells exacerbate pancreatic damage.In liver failure, the translocation of Escherichia coli causes intestinal inflammatory responses, suggesting that NOD-like receptor family protein 3 (NLPR3) may be a crucial factor in the occurrence of intestinal microbiota-related pancreatitis.Butyrate and its derived salts help protect the host’s intestines from pathogen attacks, preventing pathogenic bacteria from migrating within the intestines. The decrease in beneficial bacterial species may lead to increased permeability of the intestinal mucosa and dysfunction of the intestinal mucosal barrier, allowing pathogens to penetrate the damaged intestinal mucosa into the bloodstream, thereby promoting the development of pancreatitis.

4Oxidative Stress
The cytokines produced during liver failure are the basis of the inflammatory response. During the inflammatory response, there is an accompanying oxidative imbalance within the cells. When the balance between free radicals and the antioxidant system is disrupted, oxidative stress occurs, leading to the accumulation of specific scavenging enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GST) within the cells. During liver failure, the cytokines produced enter the pancreas, thereby inhibiting the activity of certain enzymes like SOD, CAT, and GST, obstructing energy metabolism and converting oxygen into oxygen free radicals (OFR). However, excessive accumulation of OFR may cause lipid peroxidation, calcium homeostasis disruption, and DNA damage, thus increasing pancreatic cell damage and ultimately leading to the death of pancreatic cells. Mitochondrial dysfunction leads to pancreatic stress, impaired autophagy, and lipid metabolic disorders; it also causes ROS accumulation in the body, with accumulated ROS entering the pancreas, promoting the activation of apoptosis signal-regulating kinase 1 (ASK1) under stress conditions, increasing the phosphorylation of JNK and p38 MAPK in the pancreas, which simultaneously induces high expression levels of inflammatory cytokines such as TNF-α and IL-6. Thioredoxin-interacting protein (TXNIP) is an important protein involved in the redox reactions of the inflammatory response, which regulates the inflammatory response and oxidative stress through the ASK1-dependent activation of the JNK/p38 pathway. Oxidative stress-inducible heme oxygenase-1 (HO-1) can alleviate pancreatic damage in mice by inhibiting the activation of the NF-κB pathway through its mediated anti-inflammatory and antioxidant activities. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a widely distributed transcription factor with the ability to regulate the production of antioxidants and toxic molecules within cells; during oxidative stress, the self-activation of the Nrf2-ARE pathway can enhance HO-1 activity, thereby reducing damage to the pancreas (Figure 2).
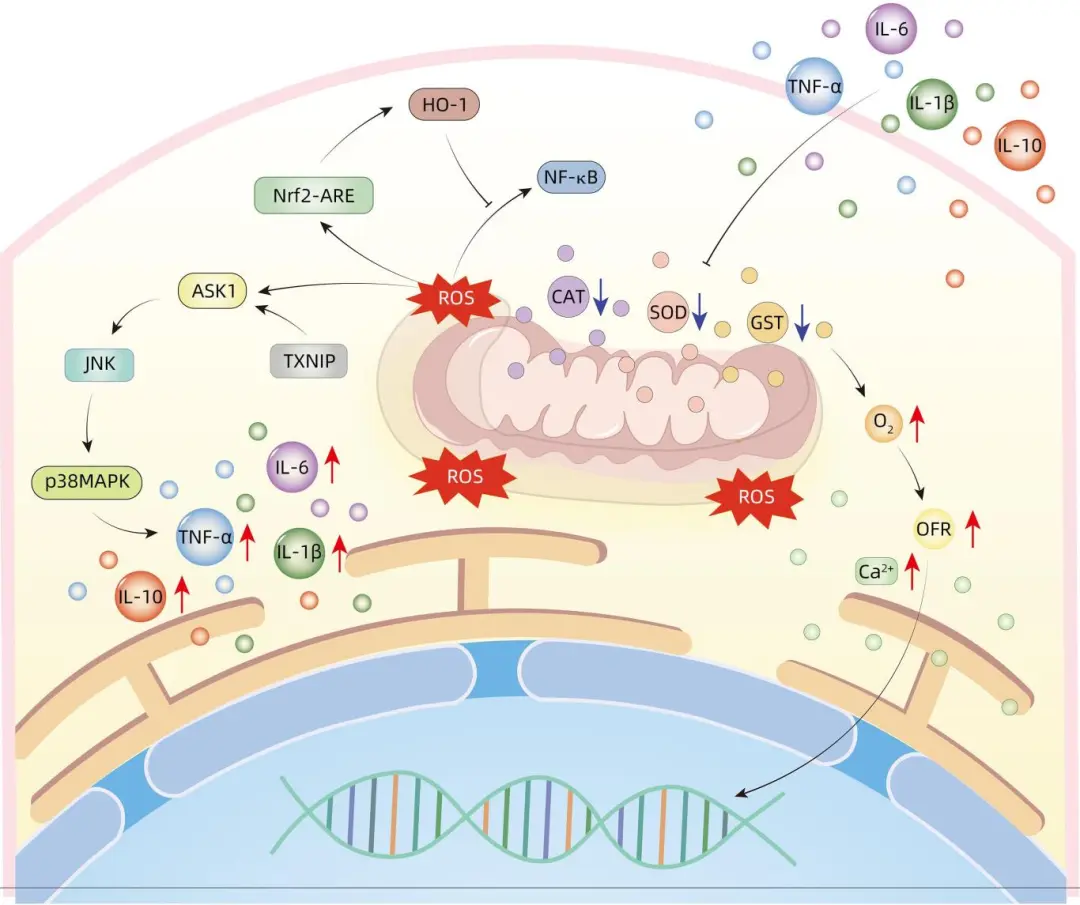
Figure 2: Mechanism of Oxidative Stress Response in Acute Pancreatitis Complicating Liver Failure

5Microcirculatory Disorders
The microcirculation of the pancreas consists of arterioles, venules, and capillary networks, influenced by the vasomotor state regulated by hormones and nerves. In pathological conditions, disturbances in pancreatic circulation usually lead to reduced tissue perfusion and ischemia, promoting the occurrence of acute pancreatitis. Endothelin-1 (ET-1) is an important factor in the microcirculatory disorders complicating acute pancreatitis in liver failure; after ET-1 binds to endothelin-A receptors, it induces strong contraction and spasm of vascular smooth muscle, leading to poor capillary perfusion, which initially causes local blood stasis and tissue ischemia and hypoxia in the pancreas. Continuous microvascular spasms will exacerbate pancreatic injury and necrosis. On the other hand, when ET-1 interacts with endothelin-A and activates the cGMP pathway, it leads to intracellular Ca2+ overload. Due to excessive calcium ions, mitochondrial energy production is insufficient, preventing the successful release of large amounts of proenzymes, which accumulate within acinar cells, thereby promoting the development of acute pancreatitis. ET can also stimulate ROS production; under inflammatory stress conditions, it participates in the activation of transcription factor NF-κB and the expression of pro-inflammatory cytokines TNF-α, IL-1, and IL-6. At the same time, damage to the endothelial cells of the pancreatic microcirculation and increased permeability further lead to ischemia and hypoxia of the pancreatic microvessels, triggering an inflammatory cascade reaction. The inflammatory response will further exacerbate the damage to endothelial cells and vascular permeability, causing blood concentration, thrombosis, and affecting blood flow perfusion in the microcirculation.

6Prevention
In previous clinical observations, the incidence of acute pancreatitis complicating liver failure is about 40%, and some patients with acute pancreatitis have relatively hidden clinical symptoms that are difficult to detect. Based on the research on the mechanisms of acute pancreatitis complicating liver failure from the five aspects mentioned above, prevention of the occurrence of acute pancreatitis in liver failure patients can be achieved through the following measures.
6.1 Active Treatment of Underlying Diseases
During the progression of liver failure, actively treating the underlying disease can prevent further deterioration of the condition and control the progression of the disease and the occurrence of complications. Intravenous infusion of albumin can regulate systemic oxidative stress and inflammation, thereby restoring immune function. Early adequate supplementation of human albumin can reduce the risk of acute pancreatitis and improve prognosis. Artificial liver plasma exchange can be used to eliminate endotoxins and inflammatory mediators as well as to replace albumin, thereby improving the survival rate of liver failure patients by inhibiting the progression of inflammatory responses and reducing the risk of acute pancreatitis. Mesenchymal stem cells can migrate to the liver through circulation, downregulating pro-inflammatory responses; they may inhibit inflammation by regulating the TLR4/NF-κBp65 pathway, reducing oxidative stress responses, thereby decreasing the incidence of acute pancreatitis. Liver transplantation remains the ultimate therapy for patients with liver failure, but due to its high cost and lack of liver donors, it is not suitable for all patients, and complications such as acute pancreatitis can easily occur after liver transplantation. A strict assessment of the final indications for liver transplantation (MELD score) and complications caused by liver cirrhosis (such as ascites and variceal bleeding) should be conducted to determine whether the patient is suitable for liver transplantation. Actively treating underlying diseases can further regulate systemic oxidative stress and inflammatory responses, restoring immune barriers and preventing the occurrence of acute pancreatitis.
6.2 Control Inflammation
The cytokines produced during liver failure are foundational for the inflammatory response. Controlling the inflammatory response during liver failure can manage inflammatory progression and prevent the onset and development of acute pancreatitis. Glucocorticoids are commonly used drugs to control inflammation progression in liver failure patients. Glucocorticoids can induce apoptosis of inflammatory cells and inhibit the presentation of antigens and the production and release of pro-inflammatory cytokines (IL-1, IL-6, TNF-α, and IL-17). At the same time, glucocorticoids have a significant stabilizing effect on cell membranes, preventing hepatocyte disintegration and necrosis, thus slowing the progression of liver damage. However, the use of glucocorticoids may increase the risk of secondary infections, dissemination, or even worsening of the primary disease, and in severe cases, sepsis may occur; it can also inhibit gastric mucus secretion, increase gastric acid and pepsin secretion, raising the likelihood of gastrointestinal ulcers, perforations, and gastrointestinal bleeding. Furthermore, due to the pharmacological characteristics of glucocorticoids, some patients may experience electrolyte, blood sugar, and blood pressure abnormalities, potentially increasing the risk of hepatic encephalopathy, hepatorenal syndrome, and other consequences of liver failure. Although glucocorticoids may increase the risk of infections and upper gastrointestinal bleeding as well as other complications, their side effects are controllable. Timely use of glucocorticoids in liver failure patients can significantly enhance survival rates; therefore, timely use of glucocorticoids can not only control the progression of systemic inflammatory responses but also further prevent the occurrence of acute pancreatitis.
6.3 Control Infection
Infection is one of the common complications of liver failure, and secondary infections in liver failure patients are primarily caused by Gram-negative bacteria. The most common sites of infection are the biliary tract, lungs, and thoracic cavity, and patients with liver failure who develop infections have an increased probability of complications, with significantly worsened prognosis. However, the clinical symptoms of infection in liver failure patients are not typical; the positive rates of procalcitonin, white blood cells, and hypersensitive C-reactive protein have high diagnostic value in cases of liver failure complicated by infection. When diagnosing and treating liver failure patients, it is important to actively recheck relevant infection indicators. Once signs of infection appear, empirical antibiotic treatment can be selected, and after relevant microbiological tests are completed, antibiotics can be adjusted based on sensitivity results.
6.4 Improve Duodenal Papilla Dysfunction
For the pathogenesis of duodenal papilla sphincter dysfunction, relieving biliary obstruction factors and alleviating biliary pressure to ensure smooth flow of bile and pancreatic juice is particularly important for the treatment of acute pancreatitis. Endoscopic retrograde cholangiopancreatography is one of the commonly used surgical methods in clinical practice; it has the advantages of combining examination and treatment with minimal trauma, effectively controlling retrograde infections of the bile duct, alleviating systemic multi-organ inflammatory responses, and also reducing the reflux of bile and pancreatic juice into the pancreatic duct, inhibiting the activation of pancreatic enzymes, and effectively controlling pancreatitis. Octreotide can significantly reduce the baseline pressure and contraction amplitude of the duodenal sphincter without affecting its contraction frequency and intraluminal pressure, reducing trypsinogen activation and pancreatic auto-digestion, thereby avoiding the occurrence of acute pancreatitis to some extent. Ursodeoxycholic acid can promote the secretion of bile acids through its protective effects on hepatocytes and cholangiocytes, improving the pressure in the duodenal sphincter, avoiding acidification in the pancreatic duct, and reducing the release of inflammatory mediators, thus preventing acute pancreatitis.
6.5 Adjust Intestinal Microbiota
In liver failure, the balance of intestinal microbiota is disrupted, and the degree of dysbiosis correlates positively with the extent of hepatocyte injury. Rifaximin, a commonly used drug for preventing intestinal infections in liver failure patients, is a broad-spectrum antibiotic that acts only in the intestines; it can adjust the imbalance of intestinal microbiota. The application of rifaximin can reduce pancreatic damage and inflammatory responses in mice with acute pancreatitis by modulating the TLR4/NF-κB inflammatory signaling pathway. Although preventive use of rifaximin may not significantly reduce the incidence of acute pancreatitis complications, it can significantly improve systemic inflammation. Bifidobacterium triple live bacteria capsules combined with enteral nutrition can actively improve the treatment effect of acute pancreatitis. In animal models of acute pancreatitis, the use of probiotics can reduce the incidence of infectious complications, improve pancreatic histological scores, and reduce mortality in rats, suggesting that it reduces the excessive growth of potential pathogenic bacteria in the duodenum, thereby decreasing the migration of intestinal bacteria to the external intestinal cavity (including the pancreas), and thus reducing infectious complications and mortality from acute pancreatitis. Actively adjusting the intestinal microbiota can prevent and mitigate pancreatic damage, avoiding the occurrence of acute pancreatitis.

7Conclusion
In clinical practice, the number of patients with liver failure complicated by acute pancreatitis has significantly increased, and the clinical manifestations of acute pancreatitis are similar to the gastrointestinal symptoms of liver failure, making it easy to overlook; without control, acute pancreatitis can lead to further damage to hepatocytes and even induce multiple organ failure. Although the specific causes of acute pancreatitis complicating liver failure are not yet fully understood, this article attempts to analyze the issue from five perspectives, indicating that these factors do not exist independently but interact and jointly promote disease progression. By proposing preventive measures for acute pancreatitis complicating liver failure from these five perspectives, it is noted that there is currently a lack of clinical and experimental research data on the prevention of acute pancreatitis complicating liver failure. According to the current state of research, clinical practice should consider the integrity of the liver, intestines, and pancreas more. During liver failure, actively treating underlying diseases, improving duodenal papilla sphincter function, adjusting intestinal microbiota, and controlling inflammation and infections are beneficial in avoiding the possibility of acute pancreatitis complicating liver failure.

https://www.lcgdbzz.org/cn/article/doi/10.12449/JCH240434
Source: Clinical Hepatobiliary Diseases Journal