
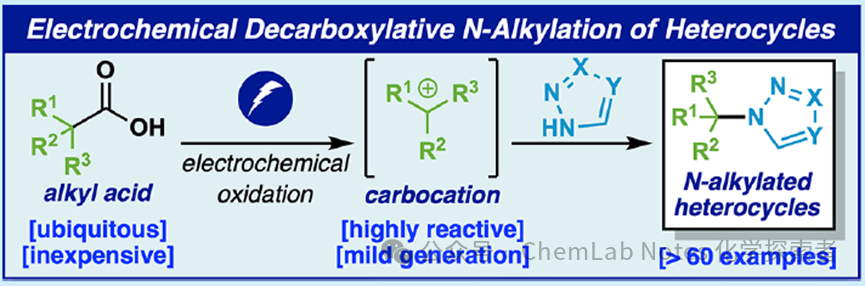
Phil S. Baran and his team have pioneered many astonishing electrocatalytic reactions with extraordinary creativity and foresight, which not only provide unprecedented green and efficient chemical synthesis but also offer scientists more elegant and sophisticated tools. His research has injected new vitality into the field of electrocatalysis, shining like a dazzling light, leading the chemistry community towards a more brilliant future.Baran and his team have developed a simple method for efficiently constructing Csp3-N bonds through an electrochemically driven anodic decarboxylation process. This method demonstrates a wide range of heterocyclic substrates (over 60 examples), significantly reducing the steps required to synthesize key target products, addressing the long-standing challenges in the synthesis of aryl N-tert-alkyl derivatives.
Previously, synthesizing the drug substrate Cereblon binder pyrazole required a tedious six-step reaction starting from 4-methylpyridine. Now, the Baran team has achieved an astonishing one-step direct synthesis of pyrazole from pyrazole (via a two-electron pathway) through clever electrocatalytic decarboxylation. After careful optimization, this N-alkylation reaction successfully yielded compound 1 with an elegant 52% isolated yield. This groundbreaking advancement not only greatly simplifies the synthetic pathway but also highlights the immense potential of electrocatalysis in modern organic synthesis.
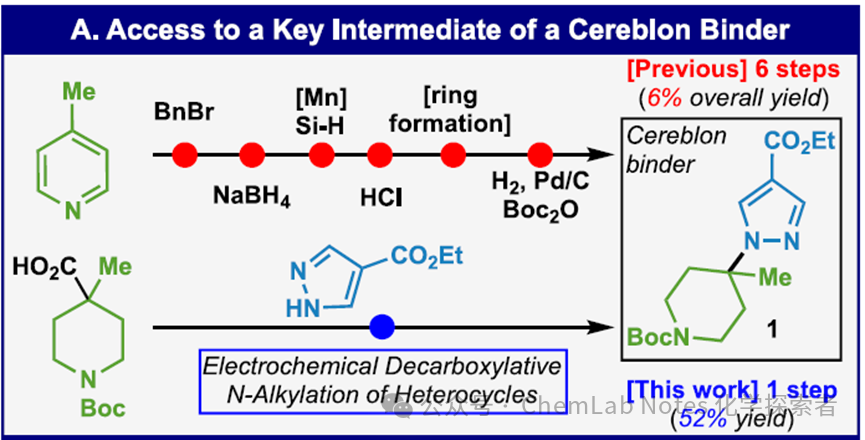
Image from Org. Lett.
The summary of the condition optimization process is as follows:
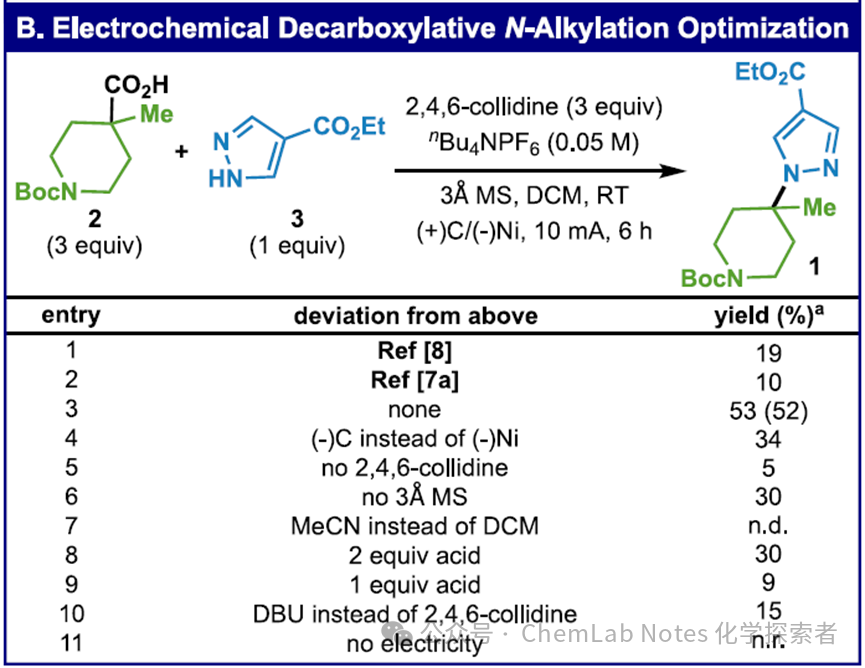 Image from Org. Lett.This condition has broad substrate adaptability, tolerating various functional groups: in addition to ester, it also includes functional groups sensitive to hydrolysis (such as nitrile), oxidation (such as BPin), reduction (such as nitro), and acidic conditions (such as acetal, Boc protected amines). Furthermore, aryl halides and fluoroalkyl substituents are also tolerated. This simple operational method (approximately 5-10 minutes setup) can be performed on a commercial potentiostat without the need to exclude air and is suitable for scale-up (for example, a yield of 69% at a 1 mmol scale) without significantly reducing the yield.
Image from Org. Lett.This condition has broad substrate adaptability, tolerating various functional groups: in addition to ester, it also includes functional groups sensitive to hydrolysis (such as nitrile), oxidation (such as BPin), reduction (such as nitro), and acidic conditions (such as acetal, Boc protected amines). Furthermore, aryl halides and fluoroalkyl substituents are also tolerated. This simple operational method (approximately 5-10 minutes setup) can be performed on a commercial potentiostat without the need to exclude air and is suitable for scale-up (for example, a yield of 69% at a 1 mmol scale) without significantly reducing the yield.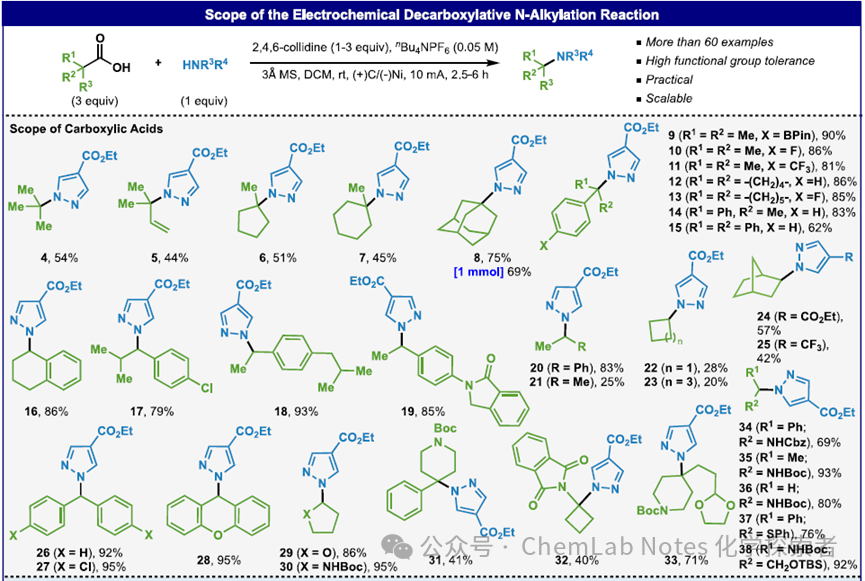 Image from Org. Lett.Regarding the applicability of heterocycles, in addition to pyrazole, (benz)triazole, tetrazole, imidazole, 1,2,4-triazole, indole, flavone, oxazolidinone, γ-lactam, succinimide, pyridinone, 2-amino pyridine, and oxazindole can also be used.
Image from Org. Lett.Regarding the applicability of heterocycles, in addition to pyrazole, (benz)triazole, tetrazole, imidazole, 1,2,4-triazole, indole, flavone, oxazolidinone, γ-lactam, succinimide, pyridinone, 2-amino pyridine, and oxazindole can also be used.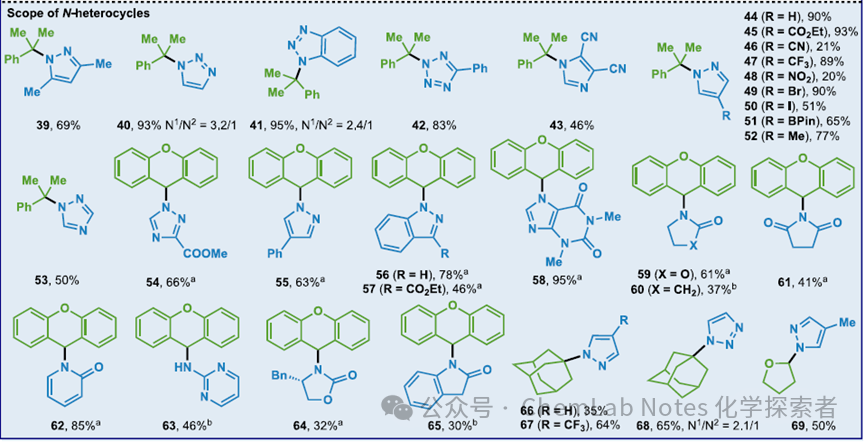 Image from Org. Lett.The method optimized by the Baran team can successfully synthesize other known compounds, such as pyrazole 70-72. Compared to the multi-step procedures used in the past, semi-acetal 70 and 71 can be obtained in high yields through a simple one-step reaction. Finally, tert-butyl pyrazole 72 can also be obtained through a one-step reaction.
Image from Org. Lett.The method optimized by the Baran team can successfully synthesize other known compounds, such as pyrazole 70-72. Compared to the multi-step procedures used in the past, semi-acetal 70 and 71 can be obtained in high yields through a simple one-step reaction. Finally, tert-butyl pyrazole 72 can also be obtained through a one-step reaction.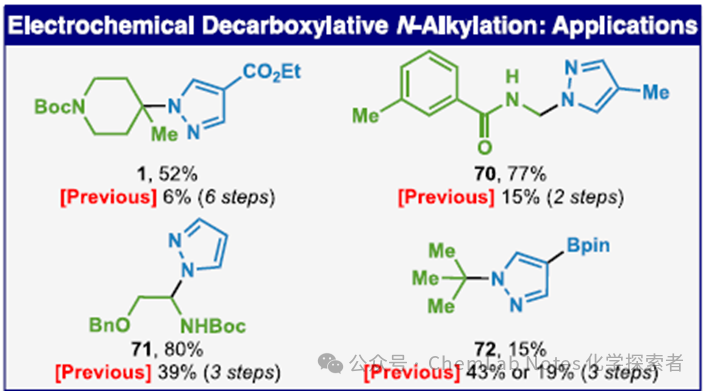 General Procedure:
General Procedure: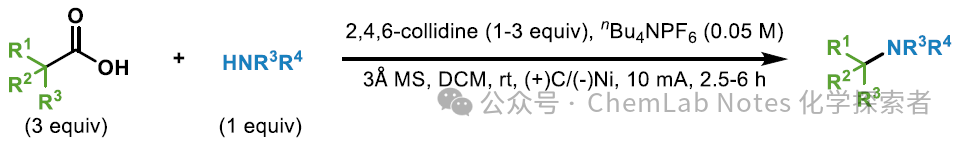
Without taking precautions to exclude air or moisture, an ElectraSyn vial (5 mL) containing a stir bar was charged with amine (0.2 mmol, 1 equiv.), carboxylic acid (0.6 mmol, 3 equiv.), nBu4NPF6 (0.15 mmol, 0.75 equiv.), 3Å molecular sieves (100 mg), 2,4,6-collidine (0.2–0.6 mmol, 1–3 equiv.), and DCM (3.0 mL). The ElectraSyn vial cap equipped with a graphite anode and a Ni cathode was inserted into the mixture. After pre-stirring for 15 minutes, the reaction mixture was electrolyzed at a constant current of 10 mA for 2.5 to 6 hours. Following electrolysis, the ElectraSyn vial cap was removed, and the electrodes were rinsed with DCM (2 mL). The resulting mixture was washed with saturated NaHCO3(aq) (10 mL), dried over Na2SO4, and concentrated in vacuo. The crude material was purified by preparative thin-layer chromatography (PTLC) to afford the desired product.
References:
https://dx.doi.org/10.1021/acs.orglett.0c02799
Note: All images in this article are sourced from the reference. If there are any copyright issues, please contact the author promptly for proper handling. If you have any questions regarding this electrochemistry, feel free to discuss with the editor.