Autoimmune encephalitis (AE) refers to encephalitis mediated by autoimmune mechanisms. Those associated with tumors are termed paraneoplastic AE, while those without tumors are classified based on the presence of autoantibodies against neuronal cell surface or synaptic proteins. Currently, clinically detectable antibodies include N-methyl-D-aspartate receptor (NMDAR) antibodies, anti-gamma-aminobutyric acid-B receptor (GABABR) antibodies, anti-leucine-rich glioma inactivated 1 protein antibodies (LGI1), anti-contactin-associated protein-like 2 (CASPR2) antibodies, and anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor 1 and 2 (AMPAR1, 2) antibodies. Among these AEs, anti-NMDAR encephalitis is the most common, accounting for about 80% of AE patients, followed by anti-LGI1 encephalitis and anti-GABABR encephalitis, while anti-CASPR2 encephalitis and anti-AMPAR encephalitis are very rare. There is a wealth of literature on anti-NMDAR encephalitis, while reports on other types of AE are relatively scarce, mostly consisting of case reports. This article summarizes the characteristics of anti-LGI1 encephalitis and anti-GABABR encephalitis treated at the First Affiliated Hospital of Zhengzhou University from June 2015 to June 2017, comparing and summarizing the findings.
Anti-LGI1 antibodies are primarily distributed in the temporal lobe cortex and hippocampus, discovered in 2010, and are part of the VGKC (voltage-gated potassium channels) complex. The common clinical features of anti-LGI1 antibody-related encephalitis include faciobrachial dystonic seizures (FBDS), recent memory decline, and hyponatremia, with tumors being rare, and immunotherapy showing good efficacy.
GABABR is located on the cell surface and is an inhibitory synaptic receptor that mediates presynaptic inhibition through at least two pathways: (1) excitation with G protein-coupled inwardly rectifying potassium channels hyperpolarizing neurons; (2) inhibiting calcium channels. GABABR can also mediate postsynaptic inhibition similarly, by reducing the firing frequency of presynaptic neurons, thereby impairing the patient’s learning and memory abilities. The clinical features of anti-GABABR antibody-related encephalitis include seizures, recent memory decline, and psychiatric behavioral abnormalities, with some patients having tumors, commonly small cell lung cancer. Immunotherapy shows good treatment effects for anti-GABABR encephalitis patients without tumors.
1. Materials and Methods
1.1 Study Subjects
A total of 2,732 autoimmune encephalitis antibody specimens were collected from the neurology department laboratory of the First Affiliated Hospital of Zhengzhou University from June 2015 to June 2017, among which 12 were positive for anti-LGI1 antibodies, accounting for 0.439%; 13 patients were positive for GABABR antibodies, accounting for 0.475%.
1.2 Autoimmune Encephalitis Antibody Testing
Indirect immunofluorescence staining kits (from Euroimmun, Germany, FAIl2d-6) were used to test the serum and cerebrospinal fluid for N-methyl-D-aspartate receptor (NMDAR) antibodies, anti-GABABR antibodies, anti-contactin-associated protein-like 2 (CASPR2) antibodies, anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor 1 and 2 (AMPAR1, 2) antibodies, and anti-leucine-rich glioma inactivated 1 protein (LGI1) antibodies.
1.3 Clinical Observation and Follow-up
Detailed data were collected from patients positive for anti-LGI1 and anti-GABABR antibodies, including clinical features, laboratory tests (blood, cerebrospinal fluid), electroencephalogram, cranial MRI, and imaging results from lung CT and PET-CT. Follow-up was mainly conducted via telephone, with some patients followed up in outpatient clinics. The diagnostic criteria for AE were based on the 2016 Lancet Neurology and the 2017 Chinese Medical Association Neurology Branch’s “Expert Consensus on the Diagnosis and Treatment of Autoimmune Encephalitis in China.” The modified Rankin Scale (mRS) was used for diagnosis and assessment before and after treatment.
2. Results
Among the 12 patients positive for LGI1 antibodies, all were male, with an average age of 62.4 years (ranging from 47 to 69 years), of which 8 were over 60 years old. Acute onset occurred in 3 cases, and subacute onset in 9 cases, with an average of 55.9 days (ranging from 23 to 130 days) from symptom onset to diagnosis. Two cases had a history of upper respiratory tract infection, and one had a history of fatigue and anger before onset. Among the 13 patients positive for GABABR antibodies, 3 were female and 10 were male, with an average onset age of 63.6 years (ranging from 37 to 73 years), of which 11 were over 60 years old. Acute onset occurred in 11 cases, and subacute onset in 2 cases, with an average of 19.9 days (ranging from 5 to 61 days) from symptom onset to diagnosis. Four cases had a history of upper respiratory tract infection, mainly presenting with fever and headache. Seven cases were associated with tumors, of which 4 had pathological results indicating small cell lung cancer.
2.1 Clinical Manifestations
Among the 12 patients with anti-LGI1 receptor encephalitis, 10 exhibited recent memory decline, 7 had decreased orientation, 9 experienced generalized tonic-clonic seizures (GTCS), 7 had FBDS, 5 had hyponatremia, 1 had psychiatric behavioral abnormalities, 1 was easily awakened, 3 had increased sleep, 4 had language disorders, 2 had choking while drinking and swallowing difficulties, 1 had headache, 2 felt dizziness, 2 had fatigue, 2 had ataxia, and 3 had bradycardia, with one patient having a pacemaker implanted due to syncope before the onset of neurological symptoms. Two patients had urinary difficulties, and one had intestinal obstruction, while another had diarrhea. Upon admission, 8 patients had an mRS score of 3, and 4 had a score of 4.
Patients with anti-GABABR encephalitis exhibited seizures in 13 cases, cognitive decline in 13 cases, psychiatric behavioral abnormalities in 11 cases, consciousness disturbances in 8 cases, fever in 8 cases, hypoventilation in 4 cases, sleep disorders in 3 cases, headache in 3 cases, aphasia in 2 cases, ataxia in 1 case, and involuntary movements of the hands in 1 case. Among them, 6 had an mRS score of 3, and 7 had severe neurological dysfunction with an mRS score of 4-5. One case was associated with interstitial lung fibrosis, while the other 6 cases were associated with lung tumors.
2.2 Tumor Associations and Others
Among the patients with anti-LGI1 encephalitis, 2 were found to have lung nodules, which showed no significant increase upon follow-up, and no biopsy was performed. One case was associated with positive thyroid antibodies and elevated rheumatoid factor IgA.
Among the 13 patients with anti-GABABR encephalitis, 8 were found to have lung masses, and 5 underwent biopsy, with 4 cases indicating small cell lung cancer and 1 case indicating right lower lung interstitial hyperplasia. One case was unstable and not suitable for biopsy, while 2 cases were discharged against medical advice, refusing biopsy. Among patients without tumors, 2 female patients had positive thyroid antibodies, and 1 male patient had elevated β2-glycoprotein 1 antibodies.
2.3 Laboratory Tests
2.3.1 Routine, Biochemical, and Cytological Examination of Cerebrospinal Fluid:
Before immunotherapy, patients with anti-LGI1 encephalitis had intracranial pressure ranging from 105 to 220 mmH2O, with 6 cases showing mild elevation of cerebrospinal fluid protein (0.48-0.58 g/L, normal range 0.15-0.45 g/L); 1 case had elevated white blood cell count (8×106/L, normal range <5×106/L), with a lymphocyte proportion of 78%. The remaining patients had normal routine, biochemical, and cytological results. Patients with anti-GABABR encephalitis had intracranial pressure ranging from 115 to 250 mmH2O, with 6 cases showing mild elevation of cerebrospinal fluid protein (0.47-0.64 g/L); 10 cases had elevated cerebrospinal fluid white blood cell counts, ranging from (6-82) ×106/L, all with increased lymphocyte proportions.
2.3.2 Autoimmune Encephalitis Antibody Testing:
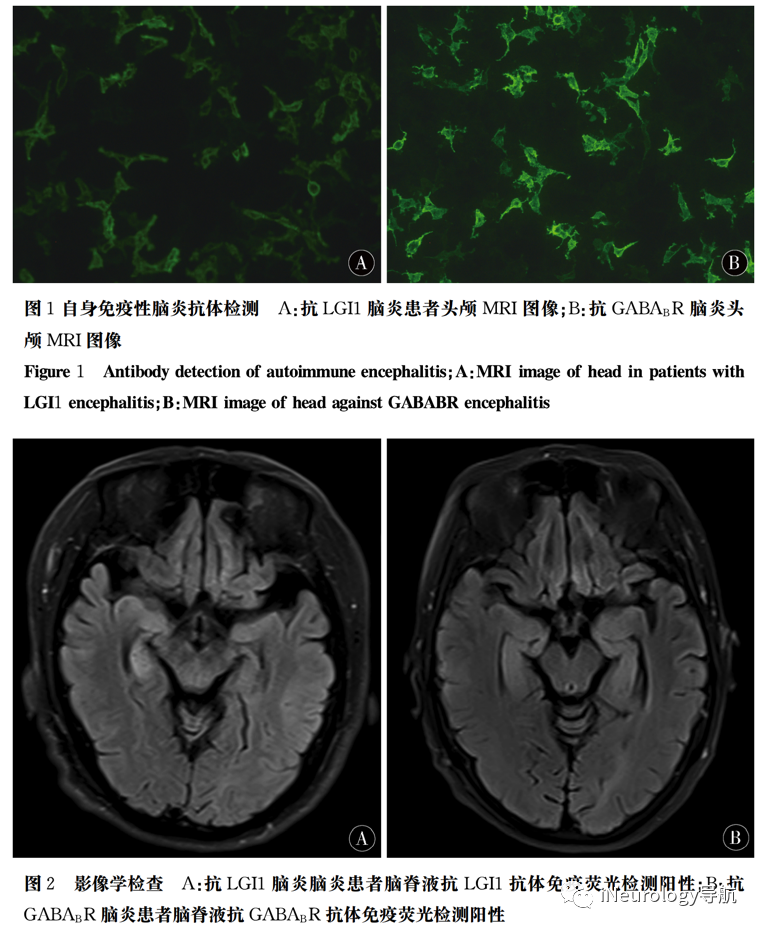
12 cases were found to be positive for anti-LGI1 antibodies, as shown in Figure 1A. 13 cases were positive for anti-GABABR antibodies (Figure 1B).
2.4 Electroencephalogram Examination
Among the 12 patients with anti-LGI1 encephalitis, 6 had abnormal electroencephalograms, while 8 of the 13 patients with anti-GABABR encephalitis had abnormal electroencephalograms, mostly showing focal or widespread slow waves, possibly accompanied by abnormal epileptic waves.
2.5 Imaging Examination
11 out of 12 patients with anti-LGI1 encephalitis underwent cranial MRI, with abnormalities found in 4 cases, affecting the medial temporal lobe (including the hippocampus) in 3 cases and the basal ganglia in 1 case (Figure 2A). One patient could not undergo MRI due to a pacemaker implantation. Among the 13 patients with anti-GABABR encephalitis, 5 had MRI abnormalities, affecting the hippocampus in 4 cases and the cerebellum in 1 case (Figure 2B).
2.6 Treatment and Prognosis
The main treatment included immunotherapy and symptomatic supportive treatment. Immunotherapy consisted of hormones and immunoglobulin, with hormone usage referring to the 2017 Chinese Medical Association Neurology Branch’s “Expert Consensus on the Diagnosis and Treatment of Autoimmune Encephalitis in China”: methylprednisolone 500 mg/d for 3 days, gradually tapering, and oral prednisone 1 mg/kg, also gradually tapering to discontinuation; intravenous immunoglobulin at 0.4 g/(kg·d) for 5 days. Among the 12 patients, 5 received combined immunoglobulin and hormone treatment, and 6 received hormone treatment alone. Other symptomatic treatments were provided for complications, such as sodium valproate, oxcarbazepine, and levetiracetam for seizure control; aripiprazole and quetiapine for psychiatric symptoms. Patients with severe hypoventilation and lung infections or status epilepticus were given tracheal intubation and ventilatory support.
Patients with anti-LGI1 encephalitis and hyponatremia were not given rapid intravenous sodium supplementation to avoid osmotic demyelination of the pons, focusing on oral sodium supplementation. One patient had normal sodium levels upon follow-up, while 5 still had low sodium levels; 3 had bradycardia, with 2 cases showing an average heart rate of >50 beats/min on dynamic electrocardiogram, asymptomatic and untreated. One case had recurrent syncope and was diagnosed with “sick sinus syndrome” before hospitalization, and after pacemaker implantation, syncope did not recur. There were no deaths among the 12 patients, and the prognosis was relatively good, with mRS scores of 2 in 2 cases, 1 in 9 cases, and 0 in 1 case, with 9 cases having residual memory impairment, 2 cases with memory impairment and drowsiness, 1 case with FBDS, and 1 case with psychiatric symptoms.
For patients with anti-GABABR encephalitis associated with primary tumors: 7 cases had lung tumors, none underwent surgical treatment, with 2 small cell lung cancer patients receiving chemotherapy, regimen: etoposide + nedaplatin. Follow-up results showed that 4 cases died, all associated with lung tumors, 1 died from status epilepticus, and 3 died from respiratory failure, none having undergone tumor intervention; 6 cases had good prognosis, all without tumors, with mRS scores of 0-2, asymptomatic or with mild cognitive decline, occasional seizures, and independent living; 3 cases had poor prognosis, still having cognitive decline and seizures, able to walk but not self-sufficient, with mRS scores of 3, all associated with lung tumors, with 2 receiving immunotherapy and tumor chemotherapy, and 1 receiving only immunotherapy.
3. Discussion
Clinical manifestations of anti-LGI1 antibody encephalitis include memory decline, seizures, and hyponatremia, with seizures commonly presenting as FBDS and generalized tonic-clonic seizures (GTCS), with various forms of seizures. FBDS can simultaneously affect the face and upper limbs, or can affect only the face, upper limbs, or lower limbs, leading to falls when affecting the lower limbs. Other types of seizures include language disturbances, loss of consciousness, memory and orientation loss, blinking, limb clonus, automatisms, shoulder shrugging, throat clearing, lip pursing, lip tremors, body shaking, and sensations of cold, heat, tingling, flushing, and fear. Some patients also exhibit autonomic nervous system symptoms such as paroxysmal tachycardia, bradycardia, sweating, diarrhea, sexual dysfunction, central hypothermia, as well as increased sleep, decreased sleep, headache, dizziness, cerebellar ataxia, and chorea. Some patients primarily present with psychiatric symptoms such as apathy, depression, anxiety, selfishness, and compulsiveness, with few seizures. In this study, one case presented with increased speech and irritability as psychiatric symptoms, with only one seizure episode, while another case presented with sequential numbness and weakness of both upper limbs and lower limbs (considered FBDS with sensory symptoms), combined with memory decline and hyponatremia, complicating diagnosis. Therefore, testing for anti-LGI1 antibodies in suspected patients is crucial for diagnosis. The sensitivity of cerebrospinal fluid is higher than that of blood; ARINO et al. tested 51 cases of anti-LGI1 antibody encephalitis blood and cerebrospinal fluid specimens, among which 47 (92%) were positive in both blood and cerebrospinal fluid, and only 4 (8%) were positive in cerebrospinal fluid alone. We observed that seizures in this disease do not respond well to antiepileptic drugs, which is a common feature of anti-LGI1 antibody encephalitis and other types of limbic encephalitis, serving as a diagnostic clue. However, immunotherapy can yield surprising effects, with significant reductions in FBDS and seizure frequency within one to several days after immunotherapy, and notable improvements in memory.
In addition to common symptoms of limbic encephalitis, autonomic nervous system involvement is also a characteristic of anti-LGI1 encephalitis. In this study, 3 patients exhibited bradycardia, with one case diagnosed as “sick sinus syndrome” due to recurrent syncope 2 months before the onset of seizures and memory impairment, treated with pacemaker implantation. About 25% of patients with anti-LGI1 encephalitis exhibit autonomic nervous system symptoms, possibly related to insular inflammation. Bowel obstruction and urinary frequency showed poor symptomatic improvement, gradually improving after immunotherapy, with no residual symptoms, indicating that the improvement of autonomic nervous system symptoms also mainly benefits from immunotherapy. Hyponatremia is also a typical manifestation of anti-LGI1 antibody encephalitis, with 5 cases exhibiting hyponatremia.
Anti-GABABR encephalitis primarily presents with seizures, cognitive decline, and psychiatric behavioral abnormalities, with some patients exhibiting strabismus, cerebellar ataxia, and brainstem encephalitis, but the onset is relatively rare. In this study, one patient had cerebellar ataxia, unsteady standing, and drunken gait, while two other patients had aphasia. Tumor association is also a characteristic of this disease, with literature reporting that about 50% of anti-GABABR encephalitis cases are associated with tumors, particularly small cell lung cancer. In this study, the average age of patients was 63.6 years, predominantly elderly males (10 cases), all with a long history of smoking, with 7 cases associated with lung tumors, 6 of which were male, suggesting a correlation between this disease and age, gender, and smoking. HOFBERGER et al. reported that idiopathic patients without tumors were predominantly female. Patients without tumors respond well to immunotherapy and have a better prognosis.
Patients with anti-GABABR encephalitis associated with tumors have a relatively poor prognosis, which depends not only on immunotherapy but also on tumor treatment. Combined tumor treatment can significantly prolong patient survival. This study found that the prognosis of patients with tumors is mainly related to the severity of symptoms at admission and whether aggressive tumor treatment and immunotherapy were performed. The 4 patients who died had mRS scores of 4-5 at admission, indicating severe illness, with 3 cases associated with hypoventilation, and none underwent tumor treatment.
The cranial MRI findings of both types of encephalitis mainly show signal abnormalities in the basal ganglia and medial temporal lobe, with follow-up revealing some patients developing hippocampal atrophy accompanied by hippocampal sclerosis, consistent with the residual memory impairment in patients. However, there are no characteristic differences in cranial MRI between the two types of encephalitis, and there are no distinctive findings in electroencephalograms or cerebrospinal fluid protein cells, thus diagnosis still relies on antibody testing for differentiation.
Overall, the prognosis of anti-LGI1 antibody encephalitis is better than that of anti-GABABR encephalitis. In this study, there were no deaths, and early immunotherapy may reduce memory impairment before severe cognitive dysfunction occurs, highlighting the importance of early diagnosis and timely immunotherapy. However, the recurrence rate of this disease is relatively high, about 27%, often occurring within 6 months after the initial onset, but can also recur years later. Recurrence and poor response to first-line immunotherapy are signs of poor prognosis, thus it is essential to avoid rapid tapering of hormones, gradually reducing them, with specific methods referring to the guidelines. If there is a poor response to first-line immunotherapy, second-line immunotherapy such as rituximab, cyclophosphamide, azathioprine, or mycophenolate mofetil can be initiated to improve prognosis. Research by GUAN et al. found that mycophenolate mofetil can improve the prognosis of anti-LGI1 antibody encephalitis.
For patients with anti-GABABR encephalitis associated with tumors, although the prognosis is poor, early detection of GABABR antibodies can facilitate early detection and treatment of tumors. Timely immunotherapy and tumor treatment can improve patient prognosis. Therefore, testing for autoimmune encephalitis antibodies is crucial, not only providing clues for the diagnosis and treatment of the disease but also determining the direction of treatment.
References
[1] VAN SONDEREN A, THIJS R D, COENDERS E C, et al. Anti-LGI1 encephalitis: Clinical syndrome and long-term follow-up. Neurology. 2016, 87 (14): 1449-1456.
[2] ARIÑO H, ARMANGUÉ T, PETIT-PEDROL M, et al. Anti-LGI1-associated cognitive impairment: Presentation and long-term outcome. Neurology, 2016, 87 (8): 759-765. doi:10.1212/WNL.0000000000003009. Epub 2016 Jul 27.
[3] SEN A, WANG J, LAUE-GIZZI H, et al. Pathognomonic seizures in limbic encephalitis associated with anti-LGI1 antibodies. Lancet, 2014, 383 (9933): 2018. doi:10.1016/S0140-6736(14)60684-X.
[4] YU J, YU X, FANG S, et al. The Treatment and Follow-Up of Anti-LGI1 Limbic Encephalitis. Eur Neurol, 2016, 75 (1/2): 5-11. doi:10.1159/000441944.
[5] Zhang Wuqiong, Yang Yu, Lan Wenjing, et al. A case report of anti-LGI1 antibody-related limbic encephalitis with positive cerebrospinal fluid herpes simplex virus antibodies. Journal of Stroke and Neurological Diseases, 2016, 33 (12): 1142-1143.
[6] PETER-DEREX L, DEVIC P, ROGEMOND V, et al. Full recovery of agrypnia associated with anti-LGI1 antibodies encephalitis under immunomodulatory treatment: a case report with sequential polysomnographic assessment. Sleep Med, 2012, 13 (5): 554-6. doi:10.1016/j.sleep.2012.01.002.
[7] LEE J J, LEE S T, JUNG K H, et al. Anti-LGI1 Limbic Encephalitis Presented with Atypical Manifestations. Exp Neurobiol, 2013, 22 (4): 337-340. doi:10.5607/en.2013.22.4.337.
[8] Nobre-Garcia A W, Gregory C P, Schlindweinzanini R, et al. Mesial temporal lobe epilepsy with hippocampal sclerosis is infrequently associated with neuronal autoantibodies. Epilepsia, 2018, 59 (9): e152-e156. doi:10.1111/epi.14534.
[9] JIA X T, PAN Y, DI Z, et al. Anti-GABA (B) receptor encephalitis in a patient with gastric adenocarcinoma. Neurol Sci, 2018: 14. doi:10.1007/s10072-018-3536-6.
[10] LI H, ZHANG A, HAO Y, et al. Coexistence of Lambert-Eaton myasthenic syndrome and autoimmune encephalitis with anti-CRMP5/CV2 and anti-GABAB receptor antibodies in small cell lung cancer: A case report. Medicine (Baltimore), 2018, 97 (19): e0696. doi:10.1097/MD.0000000000010696.
[11] MOSER A, HANSSEN H, WANDINGER K P. Excessively increased CSF glutamate levels in GABA (B) -receptor antibody associated encephalitis: A case report. J Neurol Sci, 2018, 388: 10-11. doi:10.1016/j.jns.2018.02.041.
[12] XIA J, YIN X, ZHU M, et al. Autoimmune encephalitis positive for both anti-gamma-aminobutyric acid B receptor and anti-collapsin response-mediator protein 5 antibodies: A case report. Medicine (Baltimore), 2018, 97 (3): e9574. doi:10.1097/MD.0000000000009574.
[13] GUAN W, FU Z, ZHANG H, et al. Non-tumor-Associated Anti-N-Methyl-D-Aspartate (NMDA) Receptor Encephalitis in Chinese Girls With Positive Antithyroid Antibodies. J Child Neurol, 2015, 30 (12): 1582-1585.
[14] DENG Wenjing, ZHANG Yunzhu, LI Meng, et al. Analysis of 11 cases of anti-NMDA receptor encephalitis. Chinese Journal of Practical Neurology, 2015, (14): 49-51.
[15] WU Yingying, ZHANG Jiewen. Research progress on the relationship between anti-N-methyl-D-aspartate receptor encephalitis and tumors. Chinese Journal of Neuroimmunology and Neurology, 2016, 23 (2): 125-128.
[16] JIANG Nan, GUAN Hongzhi, SUN Dawei, et al. A case of anti-N-methyl-D-aspartate receptor encephalitis combined with recurrent ovarian teratoma. Chinese Journal of Neurology, 2016, 49 (10): 789-791.
[17] THOMAS L, MAILLES A, DESESTRET V, DUCRAY F, et al. Autoimmune N-methyl-D-aspartate receptor encephalitis is a differential diagnosis of infectious encephalitis. J Infect (The Journal of infection), 2014, 68 (5): 419-425.
[18] AGAZZI P, BIEN C G, STAEDLER C, et al. Over 10-year follow-up of limbic encephalitis associated with anti-LGI1 antibodies. J Neurol, 2015, 262 (2): 469-70. doi:10.1007/s00415-014-7540-3.
[19] BYUN J I, LEE S T, MOON J, et al. Distinct intrathecal interleukin-17/interleukin-6 activation in anti-N-methyl-d-aspartate receptor encephalitis. J Neuroimmunol, 2016, 297: 141-147. doi:10.1016/j.jneuroim.2016.05.023.
[20] HU Hongtao, GUO Xiaolei, LI Mo, et al. Clinical characteristics of anti-LGI1 antibody-positive limbic encephalitis combined with Hashimoto’s encephalitis and literature review (including 1 case report). Journal of Stroke and Neurological Diseases, 2015, 32 (8): 736-739.
[21] YANG Xiaolan, LU Qinch, et al. Anti-LGI1 antibody-related limbic encephalitis primarily manifested as faciobrachial dystonic seizures: 3 case reports and literature review. Journal of Neurology and Neurorehabilitation, 2017, 13 (4): 186-196.
[22] KIM T J, LEE ST, MOON J, et al. Anti-LGI1 encephalitis is associated with unique HLA subtypes. Ann Neurol, 2017, 81 (2): 183-192. doi:10.1002/ana.24860.
[23] HOLLE J F, JESSEN F, KUHN J. Clinical Phenomenology of Autoimmune Encephalitis. Fortschr Neurol Psychiatr, 2016, 84 (5): 271-280. doi:10.1055/s-0042-104194.
[24] BEIMER N J, SELWA L M. Seizure semiology of anti-LGI1 antibody encephalitis. Epileptic Disord, 2017, 19 (4): 461-464. doi:10.1684/epd.2017.0936.
[25] CHO J J, WYMER J P. Paraneoplastic Lambert-Eaton Myasthenic Syndrome With Limbic Encephalitis: Clinical Correlation With the Coexistence of Anti-VGCC and Anti-GABAB Receptor Antibodies. J Clin Neuromuscul Dis, 2017, 19 (2): 84-88. doi:10.1097/CND.0000000000000192.
[26] CUI J, BU H, HE J, et al. The gamma-aminobutyric acid-B receptor (GABAB) encephalitis: clinical manifestations and response to immunotherapy. Int J Neurosci, 2018, 128 (7): 627-633. doi:10.1080/00207454.2017.1408618.
[27] LANCASTER E, LAI M, PENG X, et al. Antibodies to the GABA (B) receptor in limbic encephalitis with seizures: case series and characterization of the antigen. Lancet Neurol, 2010, 9 (1): 67-76.
[28] LADERA C, DEL CARMEN G M, JOSE C M, et al. Pre-synaptic GABA receptors inhibit glutamate release through GIRK channels in rat cerebral cortex. J Neurochem, 2008, 107: 1506-1517.
[29] KANEDA K, TACHIBANA Y, IMANISHI M, et al. Down-regulation of metabotropic glutamate receptor 1alpha in globus pallidus and substantia nigra of parkinsonian monkeys. Eur J Neurosci, 2005, 22: 3241-3254.
[30] NICOLL R A. My close encounter with GABA (B) receptors. Biochem Pharmacol, 2004, 68: 1667-1674.
[31] MANN E O, KOHL M M, PAULSEN O. Distinct roles of GABA (A) and GABA (B) receptors in balancing and terminating persistent cortical activity. J Neurosci, 2009, 29: 7513-7518.
[32] GRAUS F, TITULAER MJ, BALU R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol, 2016, 15 (4): 391-404.
[33] Chinese Medical Association Neurology Branch. Expert Consensus on the Diagnosis and Treatment of Autoimmune Encephalitis in China. Chinese Journal of Neurology, 2017, 50 (2): 91-98.
[34] SINGH T D, FUGATE J E, RABINSTEIN A A. The spectrum of acute encephalitis: causes, management, and predictors of outcome. Neurology, 2015, 84 (4): 359-366.
[35] GE Xiaoyan, LIU Ying, LI Yi, et al. A case report of anti-leucine-rich glioma inactivated 1 protein antibody-positive limbic encephalitis. Journal of Shandong University (Medical Edition), 2016, 54 (10): 92-94.
[36] WU Xingrao, KONG Qingxia, XIA Min. Clinical experience in the diagnosis and treatment of 10 cases of autoimmune encephalitis with seizures. Journal of Jining Medical University, 2017, 40 (5): 350-354.
[37] SUN L, CAO J, LIU C, et al. Creutzfeldt-Jakob disease versus anti-LGI1 limbic encephalitis in a patient with progressive cognitive dysfunction, psychiatric symptoms, involuntary facio-brachio-crural movement, and an abnormal electroencephalogram: a case report. Neuropsychiatr Dis Treat, 2015, 11: 1427-1430. doi:10.2147/NDT.S81414.eCollection 2015.
[38] WANG S J, ZHAO Y Y, WANG Q Z, et al. Pearls & Oysters: Limbic encephalitis associated with positive anti-LGI1 and antithyroid antibodies. Neurology, 2016, 86 (2): e16-18.
[39] TUO HZ, TIAN ZL, XUE Y, et al. Clinical analysis and literature review of 4 cases of anti-leucine-rich glioma inactivated 1 protein antibody-related limbic encephalitis. Journal of Clinical and Experimental Medicine, 2018, 17 (11): 1141-1144.
[40] HUANG Mingzhu, CHEN Lizhen, CHEN Shenggen, et al. Clinical analysis and electroencephalogram characteristics of autoimmune encephalitis. Chinese Journal of Practical Neurology, 2018, 21 (3): 273-278.
[41] AURANGZEB S, SYMMONDS M, KNIGHT R K, et al. LGI1-antibody encephalitis is characterized by frequent, multifocal clinical and subclinical seizures. Seizure, 2017, 50: 14-17.
[42] HAYASHI Y, YAMADA M, KIMURA A, et al. IVIG treatment for repeated hypothermic attacks associated with LGI1 antibody encephalitis. Neurology Neuroimmunology Neuroinflammation, 2017, 4 (4): e348.
[43] ISMAIL F S, POPKIROV S, WELLMER J, et al. Faciobrachio-crural dystonic seizures in LGI1 limbic encephalitis: A treatable cause of falls. Neurol Neuroimmunol Neuroinflamm, 2015, 2 (5): e146.
[44] TOFARIS G K, IRANI S R, CHEERAN B J, et al. Immunotherapy-responsive chorea as the presenting feature of LGI1-antibody encephalitis. Neurology, 2012, 79 (2): 195-196.
[45] YANG Xiaolan, LU Qinch, et al. Anti-LGI1 antibody-related limbic encephalitis primarily manifested as faciobrachial dystonic seizures: 3 case reports and literature review. Journal of Neurology and Neurorehabilitation, 2017, 13 (4): 186-196.
[46] YANG Yu, ZHANG Haining, CAO Jie, et al. Clinical characteristics of LGI1-Ab related limbic encephalitis (including 8 case reports and literature review). Journal of Stroke and Neurological Diseases, 2015, 32 (9): 783-787.
[47] TANG Hefei, LIU Yukun, ZHANG Ran, et al. Clinical analysis of 7 cases of anti-leucine-rich glioma inactivated 1 protein antibody-positive limbic encephalitis. Chinese Journal of Neuroimmunology and Neurology, 2015, 22 (3): 188-190.
[48] VAN SONDEREN A, THIJS R D, COENDERS E C, et al. Anti-LGI1 encephalitis: Clinical syndrome and long-term follow-up. Neurology, 2016, 87 (14): 1449-1456.
[49] ZHANG Yuanxing, YANG Huili, WU Yingying, et al. Clinical analysis of 9 cases with Anti-leucine-rich glioma inactivated 1 protein antibody associated limbic encephalitis. Chinese Medical Journal, 2017, 97 (17): 1295-1298. doi:10.3760/cma.j.issn.0376-2491.2017.17.004.
Source: Sun Guifang, Yuan Zhihao, Wang Jingtao, Peng Tao, Ma Xingrong, Gu Jinchao, Zhao Guoyou, Zhang Boai, Lu Hong. Clinical characteristics of anti-LGI1 antibody encephalitis and anti-gamma-aminobutyric acid-B receptor encephalitis. Chinese Journal of Practical Neurology, 2018, 21(16): 1759-1766.