The adrenal glands are endocrine glands that produce a variety of hormones, including adrenaline and steroid hormones such as aldosterone and cortisol. They are located above the kidneys. Each gland has an outer cortex that produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main regions: the zona glomerulosa, zona fasciculata, and zona reticularis.The adrenal cortex produces three main types of steroid hormones: mineralocorticoids, glucocorticoids, and androgens. The mineralocorticoids (such as aldosterone) produced in the zona glomerulosa help regulate blood pressure and electrolyte balance. Glucocorticoids cortisol and cortisone are synthesized in the zona fasciculata; their functions include regulating metabolism and suppressing the immune system. The innermost layer of the cortex, the zona reticularis, produces androgens, which are converted into functional sex hormones in the gonads and other target organs. The production of steroid hormones is known as steroidogenesis, involving many reactions and processes occurring in the cortical cells. The medulla produces catecholamines, which function to generate rapid responses throughout the body in stressful situations.Many endocrine disorders involve adrenal dysfunction. Excess secretion of cortisol can lead to Cushing’s syndrome, while insufficient secretion of cortisol is associated with Addison’s disease. Congenital adrenal hyperplasia is a hereditary disorder caused by a disruption in endocrine control mechanisms. Various tumors may originate from adrenal tissue and are commonly found in medical imaging when searching for other diseases.
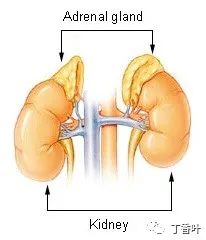
The adrenal glands are located above the kidneys.
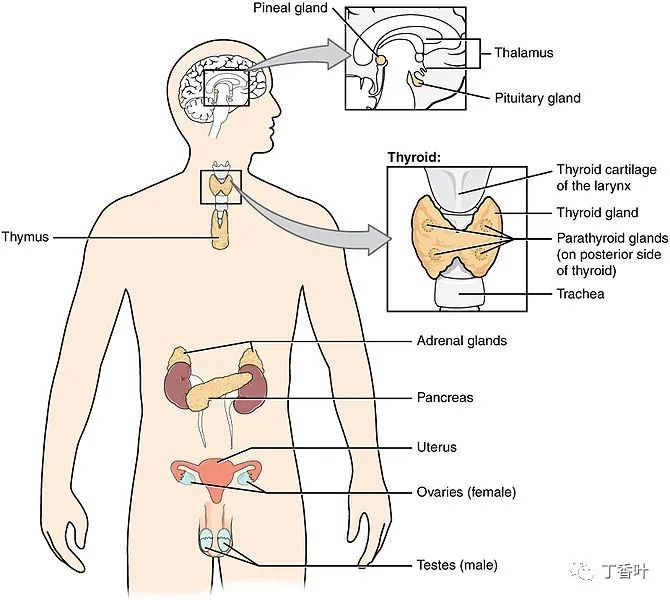
Endocrine System
1
Structure
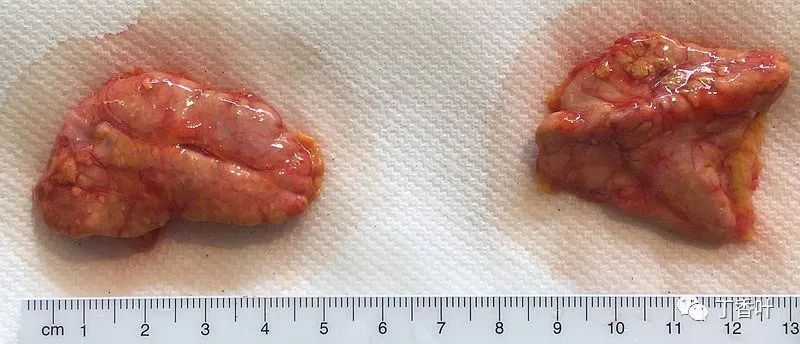
The adrenal glands, anterior (left) and posterior (right) surfaces.The adrenal glands are located retroperitoneally on either side of the body, above and slightly medial to the kidneys. In humans, the right adrenal gland is pyramid-shaped, while the left is crescent-shaped and slightly larger. The adrenal glands are approximately 3 cm wide, 5.0 cm long, and can be up to 1.0 cm thick. Their total weight in adults is 7 to 10 grams. The glands are pale yellow in color.The adrenal glands are surrounded by a fatty capsule, located within the renal fascia, which also surrounds the kidneys. A thin connective tissue septum separates the glands from the kidneys. The adrenal glands are directly located beneath the diaphragm and are connected to the diaphragm’s crura via the renal fascia.Each adrenal gland has two distinct parts, each with unique functions: the outer adrenal cortex and the inner medulla, both of which produce hormones.
2
Adrenal Cortex
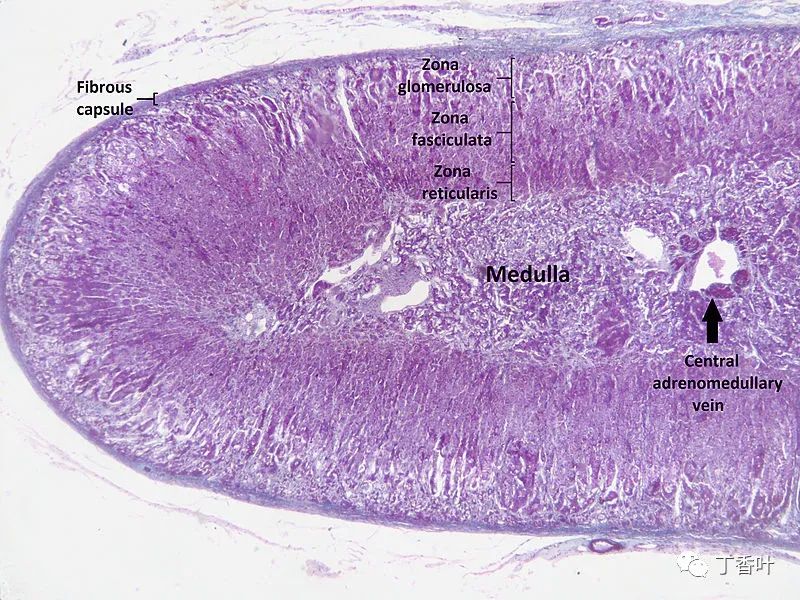
Microscopic section of the human adrenal gland showing its different layers. From the surface to the center: zona glomerulosa, zona fasciculata, zona reticularis, and medulla. In the medulla, the central adrenal medullary vein is visible.The adrenal cortex is the outer region and the largest part of the adrenal gland. It is divided into three distinct zones: the zona glomerulosa, zona fasciculata, and zona reticularis. Each zone is responsible for producing specific hormones. The adrenal cortex is the outermost layer of the adrenal gland. Within the cortex, there are three layers, referred to as “zones.” When observed under a microscope, each layer has a different appearance and function. The adrenal cortex is dedicated to producing hormones, namely aldosterone, cortisol, and androgens.
3
Zona Glomerulosa
The outermost layer of the adrenal cortex is the zona glomerulosa. It is located directly beneath the fibrous capsule of the gland. The cells in this layer form oval clusters separated by fine connective tissue strands and are associated with wide capillaries.This layer is the primary site for the production of aldosterone (a mineralocorticoid) through the action of aldosterone synthase. Aldosterone plays a crucial role in the long-term regulation of blood pressure.
4
Zona Fasciculata
The zona fasciculata is located between the zona glomerulosa and the zona reticularis. The cells in this layer are responsible for producing glucocorticoids, such as cortisol. It is the largest of the three layers, accounting for nearly 80% of the cortex’s volume. In the zona fasciculata, the cells are arranged in radial columns toward the medulla. The cells contain numerous lipid droplets, abundant mitochondria, and a complex smooth endoplasmic reticulum.
5
Zona Reticularis
The innermost cortical layer, the zona reticularis, is directly adjacent to the medulla. It produces androgens in the human body, primarily dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and androstenedione (a precursor to testosterone). Its small cells form irregular cords and clusters, separated by capillaries and connective tissue. The cells contain relatively little cytoplasm and lipid droplets, sometimes showing brown lipofuscin.
6
Medulla
The adrenal medulla is located at the center of each adrenal gland, surrounded by the adrenal cortex. The chromaffin cells of the medulla are the primary source of catecholamines (such as adrenaline and noradrenaline) released into the body. Approximately 20% of norepinephrine (noradrenaline) and 80% of epinephrine (adrenaline) are secreted here.The adrenal medulla is driven by the sympathetic nervous system through preganglionic fibers originating from the thoracic spinal cord, specifically from vertebrae T5-T11. Because it is innervated by preganglionic nerve fibers, the adrenal medulla can be considered a specialized sympathetic ganglion. However, unlike other sympathetic ganglia, the adrenal medulla lacks distinct synapses and releases its secretions directly into the bloodstream.
7
Blood Supply
The adrenal glands are among the organs with the highest blood supply per gram of tissue: each gland can have up to 60 small arteries. Three arteries typically supply each adrenal gland:Adrenal artery, a branch of the inferior phrenic arteryAdrenal artery, a direct branch of the abdominal aortaAdrenal artery, a branch of the renal arteryThese vessels supply a network of small arteries within the adrenal capsule. Fine strands from the capsule enter the gland, delivering blood to the gland.Venous blood is drained from the gland via the adrenal vein, usually one per gland:The right adrenal vein drains into the inferior vena cavaThe left adrenal vein drains into the left renal vein or left inferior phrenic vein.The central adrenal medullary vein in the adrenal medulla is an unusual vessel. Its structure differs from other veins in that the tunica media (the middle layer of the vessel) contains smooth muscle arranged in distinct longitudinal bundles.
8
Variability
The adrenal glands may be underdeveloped or may fuse at the midline behind the aorta. These are associated with other congenital anomalies, such as renal agenesis or renal fusion. The glands may develop with partial or complete absence of the cortex or may develop in unusual locations.
9
Function
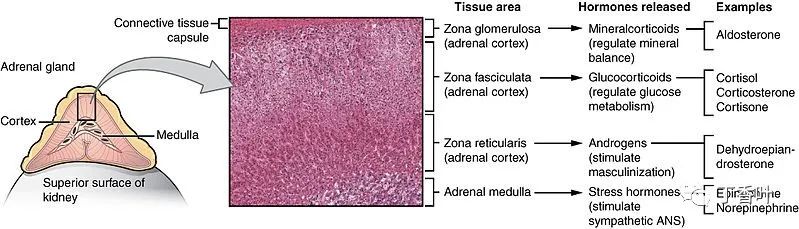
Different hormones are produced in different regions of the cortex and medulla of the gland. Optical microscope at ×204 magnification.The adrenal glands secrete many different hormones, which are metabolized by enzymes either within the gland or in other parts of the body. These hormones are involved in many essential biological functions.
10
Corticosteroids
Corticosteroids are a group of steroid hormones produced by the adrenal cortex, hence the name.Mineralocorticoids such as aldosterone regulate salt (“mineral”) balance and blood volume.Glucocorticoids such as cortisol affect the metabolic rates of proteins, fats, and sugars (“glucose”).Androgens such as dehydroepiandrosterone.
11
Mineralocorticoids
The adrenal glands produce aldosterone, a mineralocorticoid that is important in regulating salt (“mineral”) balance and blood volume. In the kidneys, aldosterone acts on the distal convoluted tubules and collecting ducts by increasing sodium reabsorption and the excretion of potassium and hydrogen ions. Aldosterone is responsible for the reabsorption of about 2% of the filtered glomerular filtrate. Sodium retention is also a response of the distal colon and sweat glands to aldosterone receptor stimulation. Angiotensin II and extracellular potassium are the two main regulators of aldosterone production. The amount of sodium in the body affects extracellular volume, which in turn affects blood pressure. Therefore, the role of aldosterone in sodium retention is crucial for blood pressure regulation.
12
Glucocorticoids
Cortisol is the primary glucocorticoid in humans. In species that do not produce cortisol, this role is taken over by corticosterone. Glucocorticoids have many effects on metabolism. As the name suggests, they increase circulating levels of glucose. This is due to increased mobilization of amino acids from proteins and stimulation of the liver to synthesize glucose from these amino acids. Additionally, they increase levels of free fatty acids, which cells can use as an alternative energy source instead of glucose. Glucocorticoids also have effects unrelated to regulating blood sugar levels, including suppression of the immune system and effective anti-inflammatory actions. Cortisol reduces the ability of osteoblasts to produce new bone tissue and decreases gastrointestinal absorption of calcium.The adrenal glands secrete a baseline level of cortisol but can also produce large amounts of the hormone in response to adrenocorticotropic hormone (ACTH) from the anterior pituitary. Cortisol is not released evenly throughout the day—due to the circadian rhythm of ACTH secretion, its concentration in the blood is highest in the morning and lowest in the evening. Cortisone is an inactive product of the action of the enzyme 11β-HSD on cortisol. The reaction catalyzed by 11β-HSD is reversible, meaning it can convert administered cortisone into the biologically active hormone cortisol.
13
Formation
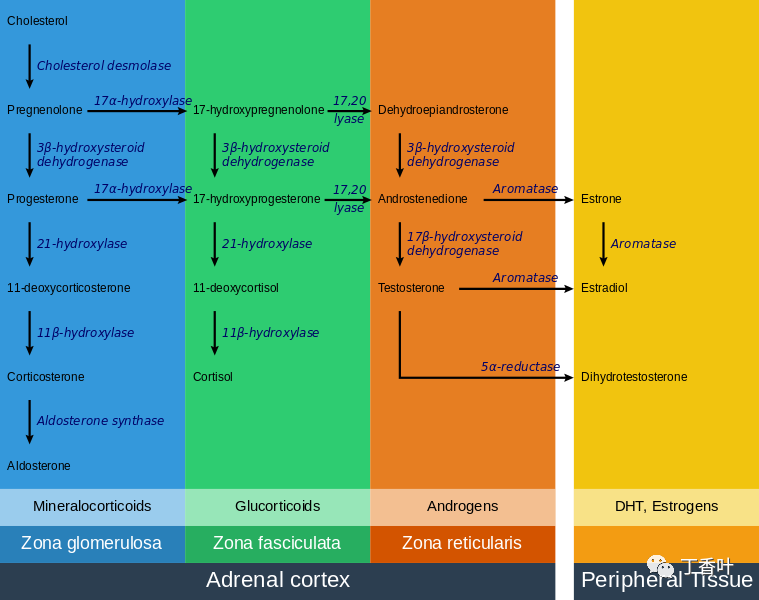
Steroidogenesis in the adrenal gland—different steps occur in different layers of the glandAll corticosteroid hormones share cholesterol as a common precursor. Therefore, the first step in steroidogenesis is the uptake or synthesis of cholesterol. The cells that produce steroid hormones can obtain cholesterol through two pathways. The primary source is dietary cholesterol transported in the blood as cholesterol esters within low-density lipoprotein (LDL). Low-density lipoprotein enters the cells via receptor-mediated endocytosis. Another source of cholesterol is synthesis within the endoplasmic reticulum of the cell. When LDL levels are abnormally low, synthesis can compensate. In lysosomes, cholesterol esters are converted to free cholesterol, which is then used for steroidogenesis or stored within the cell.The initial part of the conversion of cholesterol to steroid hormones involves many enzymes from the cytochrome P450 family located in the inner mitochondrial membrane. The transport of cholesterol from the outer membrane to the inner membrane is facilitated by steroidogenic acute regulatory protein and is the rate-limiting step in steroid synthesis.The different layers of the adrenal gland have different functions, each with distinct enzymes that produce different hormones from a common precursor. The first enzymatic step in producing all steroid hormones is the cleavage of the cholesterol side chain, a reaction catalyzed by the enzyme P450scc (also known as cholesterol side-chain cleavage enzyme), resulting in the formation of pregnenolone. Once pregnenolone is produced, specific enzymes in each cortical layer further modify it. The enzymes involved in this process include mitochondrial and microsomal P450s and hydroxysteroid dehydrogenases. Many intermediate steps are typically required, during which pregnenolone is modified multiple times to form functional hormones. The enzymes that catalyze reactions in these metabolic pathways are involved in many endocrine diseases. For example, the most common form of congenital adrenal hyperplasia is due to a deficiency of 21-hydroxylase, an enzyme involved in the intermediate steps of cortisol production.
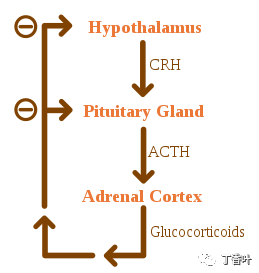
Negative feedback of the HPA axisGlucocorticoids are regulated by the hypothalamic-pituitary-adrenal (HPA) axis. The synthesis of glucocorticoids is stimulated by adrenocorticotropic hormone (ACTH), a hormone released into the bloodstream by the anterior pituitary. In turn, the presence of corticotropin-releasing hormone (CRH) stimulates the production of ACTH, which is released by hypothalamic neurons. ACTH acts on adrenal cells primarily by increasing intracellular levels of StAR, followed by all the P450 enzymes involved in steroidogenesis. The HPA axis is an example of a negative feedback system, where cortisol itself acts as a direct inhibitor of CRH and ACTH synthesis. The HPA axis also interacts with the immune system by increasing ACTH secretion in the presence of certain molecules during inflammatory responses.The secretion of mineralocorticoids is primarily regulated by the renin-angiotensin-aldosterone system (RAAS), potassium concentration, and to a lesser extent, ACTH levels. Blood pressure sensors in the juxtaglomerular apparatus of the kidneys release renin into the bloodstream, initiating a cascade of reactions that lead to the formation of angiotensin II. The angiotensin receptors in the zona glomerulosa cells recognize this substance and stimulate the release of aldosterone upon binding.
14
Androgens
Cells in the zona reticularis of the adrenal gland produce male hormones or androgens, the most important of which is DHEA. Generally, these hormones have no overall effect on the male body, as they are converted in the gonads into more potent androgens such as testosterone and DHT, or converted into estrogens (female sex hormones), serving as metabolic intermediates.
15
Catecholamines
Commonly referred to in the United States as adrenaline and noradrenaline, adrenaline and noradrenaline are catecholamines, water-soluble compounds characterized by a catechol group and an amine group. The adrenal glands are responsible for most of the body’s circulating adrenaline but only a small amount of circulating noradrenaline. These hormones are released by the adrenal medulla, which contains a dense network of blood vessels. Adrenaline and noradrenaline act on adrenergic receptors throughout the body, with effects including increased blood pressure and heart rate. The actions of adrenaline and noradrenaline are responsible for the fight-or-flight response, characterized by increased respiration and heart rate, elevated blood pressure, and vasoconstriction in many parts of the body.
16
Structure
Catecholamines are produced in the chromaffin cells of the adrenal medulla from tyrosine, a non-essential amino acid derived from food, or produced from phenylalanine in the liver. Tyrosine hydroxylase converts tyrosine into L-DOPA in the first step of catecholamine synthesis. L-DOPA is then converted into dopamine before being converted into noradrenaline in the cytosol. In the cytoplasm, noradrenaline is converted into adrenaline by phenylethanolamine N-methyltransferase (PNMT) and stored in granules. Glucocorticoids produced in the adrenal cortex stimulate catecholamine synthesis by increasing the levels of tyrosine hydroxylase and PNMT.The release of catecholamines is stimulated by activation of the sympathetic nervous system. The visceral nerves of the sympathetic nervous system innervate the adrenal medulla. When activated, it causes the release of catecholamines from storage granules by stimulating the opening of calcium channels in the cell membrane.
17
Gene and Protein Expression
The human genome includes approximately 20,000 protein-coding genes, of which 70% are expressed in the normal adult adrenal gland. Compared to other organs and tissues, only about 250 genes are more specifically expressed in the adrenal gland. The adrenal-specific genes with the highest expression levels include members of the cytochrome P450 enzyme superfamily. Corresponding proteins are expressed in different compartments of the adrenal gland, such as CYP11A1, HSD3B2, and FDX1, which are involved in steroid hormone synthesis and expressed in the cortical cell layers, as well as PNMT and DBH, which are involved in the synthesis of noradrenaline and adrenaline and expressed in the medulla.
18
Development
The adrenal glands consist of two heterogeneous types of tissue. The inner part is the adrenal medulla, which produces adrenaline and noradrenaline and releases them into the bloodstream as part of the sympathetic nervous system. Surrounding the medulla is the cortex, which produces various steroid hormones. These tissues arise from different embryonic precursors and have different prenatal developmental pathways. The adrenal cortex originates from the mesoderm, while the medulla originates from the neural crest, which is derived from the ectoderm.The adrenal glands of newborns are proportionally much larger than those of adults. For example, at three months of age, the glands are four times the size of the kidneys. The size of the glands decreases relative to the body after birth, primarily due to the contraction of the cortex. The cortex nearly completely disappears by age one and re-develops again by ages four to five. The glands weigh about 1 gram at birth and about 4 grams each in adulthood. The glands can first be detected after the sixth week of fetal development.
19
Cortex
The adrenal cortex tissue originates from the intermediate mesoderm. It first appears 33 days after fertilization, shows the ability to produce steroid hormones by the 8th week, and grows rapidly during the first three months of pregnancy. The fetal adrenal cortex differs from its adult counterpart as it consists of two distinct regions: an inner “fetal” region, which carries out most of the hormone-producing activity, and an outer “definitive” region, which is in a proliferative state. The fetal zone produces large amounts of adrenal androgens (male hormones), which the placenta uses for estrogen biosynthesis. The development of the adrenal cortex is primarily regulated by adrenocorticotropic hormone (ACTH), a hormone produced by the pituitary gland that stimulates cortisol synthesis. During mid-pregnancy, the fetal zone occupies most of the cortex’s volume and produces 100-200 mg/day of DHEA-S, a precursor to androgens and estrogens (female sex hormones). Adrenal hormones, especially glucocorticoids like cortisol, are crucial for the prenatal development of organs, particularly lung maturation. Due to the rapid disappearance of the fetal zone, the adrenal glands decrease in size after birth, and androgen secretion correspondingly decreases.
20
Adrenaline
In early childhood, androgen synthesis and secretion remain low, but changes in the anatomy and function of cortical androgen production occur in the years leading up to puberty (ages 6-8), leading to increased secretion of the steroids DHEA and DHEA-S. These changes are part of the process of adrenal puberty, which has only been described in humans and some other primates. Adrenal puberty is independent of ACTH or gonadotropin and is associated with the gradual thickening of the zona reticularis layer. Functionally, adrenal puberty provides a source of androgens for the development of axillary and pubic hair before the onset of puberty.
21
Medulla
The adrenal medulla originates from neural crest cells, which are derived from the ectoderm of the embryo. These cells migrate from their initial location and cluster near the dorsal aorta, a primitive blood vessel, activating their differentiation by releasing proteins known as BMP. These cells then undergo a second migration from the dorsal aorta, forming the adrenal medulla and other organs of the sympathetic nervous system. The cells of the adrenal medulla are called chromaffin cells because they contain granules that stain with chromium salts, a feature not present in all sympathetic organs. Glucocorticoids produced in the adrenal cortex were once thought to be the cause of chromaffin cell differentiation. Recent studies suggest that BMP-4 secreted in the adrenal tissue is the primary cause, while glucocorticoids play a role only in the subsequent development of the cells.
22
Clinical Significance
The normal function of the adrenal glands can be impaired by diseases such as infections, tumors, genetic disorders, and autoimmune diseases, as well as side effects of drug treatments. These diseases directly affect the glands (such as infections or autoimmune diseases) or lead to excess or deficiency of adrenal hormones due to hormonal production disorders (such as certain types of Cushing’s syndrome) and associated symptoms.
23
Excess Production of Corticosteroids
Cushing’s Syndrome
Cushing’s syndrome is characterized by an excess of glucocorticoids. It may result from long-term treatment with glucocorticoids or from underlying diseases that cause changes in the HPA axis or cortisol production. The causes can be further divided into ACTH-dependent or ACTH-independent. The most common cause of endogenous Cushing’s syndrome is a pituitary adenoma that leads to excessive production of ACTH. This condition produces various signs and symptoms, including obesity, diabetes, hypertension, hirsutism (excessive hair growth), osteoporosis, depression, and most notably, the appearance of striae due to thinning of the skin.
24
Primary Aldosteronism
Primary aldosteronism results when the zona glomerulosa produces excess aldosterone. The causes of this condition are bilateral hyperplasia of the gland (overgrowth of tissue) or an aldosterone-producing adenoma (a condition known as Conn’s syndrome). Primary aldosteronism leads to hypertension and electrolyte imbalances, increasing potassium depletion and sodium retention.
25
Adrenal Insufficiency
Approximately 5 out of every 10,000 people in the general population have adrenal insufficiency (glucocorticoid deficiency). Diseases classified as primary adrenal insufficiency (including Addison’s disease and genetic causes) directly affect the adrenal cortex. If problems affecting the hypothalamic-pituitary-adrenal axis occur outside the gland, it is termed secondary adrenal insufficiency.
26
Addison’s Disease
Characteristic skin hyperpigmentation in Addison’s diseaseAddison’s disease refers to primary adrenal insufficiency, which is characterized by a deficiency of glucocorticoids and mineralocorticoids produced by the adrenal glands. In the Western world, Addison’s disease is most commonly an autoimmune disease in which the body produces antibodies against adrenal cortical cells. Globally, this disease is more commonly caused by infections, particularly tuberculosis. A notable feature of Addison’s disease is skin hyperpigmentation, along with other nonspecific symptoms such as fatigue.Complications that arise in untreated Addison’s disease and other types of primary adrenal insufficiency include adrenal crisis, a medical emergency in which low levels of glucocorticoids and mineralocorticoids lead to hypovolemic shock and symptoms such as vomiting and fever. An adrenal crisis can gradually lead to stupor and coma. Management of an adrenal crisis includes the injection of hydrocortisone.
27
Secondary Adrenal Insufficiency
In secondary adrenal insufficiency, dysfunction of the hypothalamic-pituitary-adrenal axis leads to reduced stimulation of the adrenal cortex. Besides suppression of the axis due to glucocorticoid treatment, the most common cause of secondary adrenal insufficiency is tumors affecting the pituitary that produce adrenocorticotropic hormone (ACTH). This type of adrenal insufficiency typically does not affect the production of mineralocorticoids, which are regulated by the renin-angiotensin system.
28
Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia is a congenital disorder in which mutations in enzymes that produce steroid hormones lead to glucocorticoid deficiency and dysfunction of the negative feedback loop of the HPA axis. In the HPA axis, cortisol (a glucocorticoid) inhibits the release of CRH and ACTH, which in turn stimulate the synthesis of corticosteroids. Due to the inability to synthesize cortisol, these hormones are released in large amounts, stimulating the production of other adrenal steroids. The most common form of congenital adrenal hyperplasia is due to a deficiency of 21-hydroxylase. 21-hydroxylase is necessary for the production of mineralocorticoids and glucocorticoids but not androgens. Therefore, ACTH stimulation of the adrenal cortex leads to excessive release of adrenal androgens, resulting in ambiguous genitalia and the development of secondary sexual characteristics.
29
Adrenal Tumors
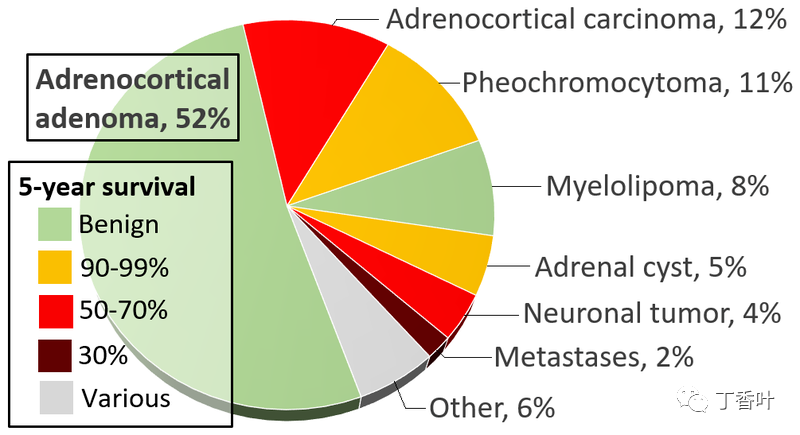
Incidence and prognosis of adrenal tumors.Adrenal tumors are often found as incidentalomas, which are asymptomatic tumors discovered during medical imaging. They appear in approximately 3.4% of CT scans, and in most cases, they are benign adenomas. Adrenal cancer is very rare, with an incidence of one in a million per year.Pheochromocytomas are tumors of the adrenal medulla that originate from chromaffin cells. They produce a variety of nonspecific symptoms, including headaches, sweating, anxiety, and palpitations. Common signs include hypertension and tachycardia. Surgery, especially laparoscopic adrenalectomy, is the most commonly used method for treating small pheochromocytomas.
30
History
Bartolomeo Eustachi was an Italian anatomist who first described the adrenal glands in 1563-4. However, these publications were part of the papal library and did not receive public attention until 1611 when they were received with illustrations by elder Caspar Bartholin.The adrenal glands are named for their position relative to the kidneys. The term “adrenal” comes from ad- (Latin for “near”) and renes (Latin for “kidneys”). Similarly, “suprarenal,” named by Jean Riolan the Younger in 1629, derives from the Latin supra (Latin for “above”) and renes (Latin for “kidneys”). It was not until the 19th century that the adrenal nature of the glands was truly accepted, as anatomists elucidated the gland’s ductless nature and its possible secretory function—prior to this, there was debate about whether the glands were indeed adrenal or partially renal.In 1855, British physician Thomas Addison published “On the Constitutional and Local Effects of Disease of the Suprarenal Capsules,” one of the most recognized works on the adrenal glands. In his treatise, Addison described what would later be named Addison’s disease by French physician Georges Trousseau, a name still used today for adrenal insufficiency and its associated clinical manifestations. In 1894, British physiologists George Oliver and Edward Schafer studied the effects of adrenal extracts and observed their hypertensive effects. In the following decades, several physicians attempted to treat Addison’s disease with adrenal cortex extracts. Edward Calvin Kendall, Philip Hench, and Tadeusz Reichstein subsequently received the Nobel Prize in Physiology or Medicine in 1950 for their discoveries regarding the structure and function of adrenal hormones.References Santulli G. MD (2015). Adrenal Glands: From Pathophysiology to Clinical Evidence. Nova Science Publishers, New York, NY. ISBN 978-1-63483-570-1. “Adrenal gland”. Medline Plus/Merriam-Webster Dictionary. Retrieved 11 February 2015. Ross M, Pawlina W (2011). Histology: A Text and Atlas (6th ed.). Lippincott Williams & Wilkins. pp. 708, 780. ISBN 978-0-7817-7200-6. Melmed, S; Polonsky, KS; Larsen, PR; Kronenberg, HM (2011). Williams Textbook of Endocrinology (12th ed.). Saunders. ISBN 978-1437703245. Miller, WL; Auchus, RJ (2011). “The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders”. Endocrine Reviews. 32 (1): 81–151. doi:10.1210/er.2010-0013. PMC 3365799. PMID 21051590. Longo, D; Fauci, A; Kasper, D; Hauser, S; Jameson, J; Loscalzo, J (2012). Harrison’s Principles of Internal Medicine (18th ed.). New York: McGraw-Hill. ISBN 978-0071748896. Nieman, LK (2010). “Approach to the patient with an adrenal incidentaloma”. The Journal of Clinical Endocrinology and Metabolism. 95 (9): 4106–13. doi:10.1210/jc.2010-0457. PMC 2936073. PMID 20823463. Thomas, edited by Paul; Molecular, School of; Australia, Biomedical Science, University of Adelaide, Adelaide, South Australia (2013). Endocrine Gland Development and Disease. Burlington: Elsevier Science. p. 241. ISBN 9780123914545. Antonio Carlos A. Westphalen and Bonnie N. Joe (2006). “CT and MRI of Adrenal Masses”. Appl Radiol. 35 (8): 10–26. O’Hare, A. Munro Neville, Michael J. (1982). The Human Adrenal Cortex Pathology and Biology – An Integrated Approach. Springer London. pp. Chapter 4: Structure of the adult cortex. ISBN 9781447113171. Moore KL, Dalley AF, Agur AM (2013). Clinically Oriented Anatomy, 7th ed. Lippincott Williams & Wilkins. pp. 294, 298. ISBN 978-1-4511-8447-1. Kay, Saundra. “Adrenal Glands”. Medscape. Retrieved 1 August 2015. Whitehead, Saffron A.; Nussey, Stephen (2001). Endocrinology: an integrated approach. Oxford: BIOS. p. 122. ISBN 978-1-85996-252-7. Jefferies, William McK (2004). Safe uses of cortisol. Springfield, Ill: Charles C. Thomas. ISBN 978-0-398-07500-2. Young B, Woodford P, O’Dowd G (2013). Wheater’s Functional Histology: A Text and Colour Atlas (6th ed.). Elsevier. p. 329. ISBN 978-0702047473. Curnow KM, Tusie-Luna MT, Pascoe L, Natarajan R, Gu JL, Nadler JL, White PC (October 1991). “The product of the CYP11B2 gene is required for aldosterone biosynthesis in the human adrenal cortex” (PDF). Mol. Endocrinol. 5 (10): 1513–1522. doi:10.1210/mend-5-10-1513. PMID 1775135. Zhou M, Gomez-Sanchez CE (July 1993). “Cloning and expression of a rat cytochrome P-450 11 beta-hydroxylase/aldosterone synthase (CYP11B2) cDNA variant”. Biochem Biophys Res Commun. 194 (1): 112–117. doi:10.1006/bbrc.1993.1792. PMID 8333830. Marieb, EN; Hoehn, K (2012). Human anatomy & physiology (9th ed.). Pearson. p. 629. ISBN 978-0321743268. Dunn R. B.; Kudrath W.; Passo S.S.; Wilson L.B. (2011). “10”. Kaplan USMLE Step 1 Physiology Lecture Notes. pp. 263–289. Sapru, Hreday N.; Siegel, Allan (2007). Essential Neuroscience. Hagerstown, MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-9121-2. Mirilas P, Skandalakis JE, Colborn GL, Weidman TA, Foster RS, Kingsnorth A, Skandalakis LJ, Skandalakis PN (2004). Surgical Anatomy: The Embryologic And Anatomic Basis Of Modern Surgery. McGraw-Hill Professional Publishing. ISBN 978-960-399-074-1. “OpenStax CNX”. cnx.org. Retrieved 1 August 2015. Britton, the editors Nicki R. Colledge, Brian R. Walker, Stuart H. Ralston; illustrated by Robert (2010). Davidson’s principles and practice of medicine (21st ed.). Edinburgh: Churchill Livingstone/Elsevier. pp. 768–778. ISBN 978-0-7020-3085-7. “Corticosteroid”. TheFreeDictionary. Retrieved 23 September 2015. Marieb Human Anatomy & Physiology 9th edition, chapter:16, page:629, question number:14 “Corticosteroid”. TheFreeDictionary. Retrieved 23 September 2015. Sherwood, Lauralee (2001). Human physiology: from cells to systems. Pacific Grove, CA: Brooks/Cole. ISBN 978-0-534-56826-9. OCLC 43702042. Boron, WF.; Boulapep, EL. (2012). Medical Physiology (2nd ed.). Philadelphia: Saunders. ISBN 978-1437717532. Miller, WL; Bose, HS (2011). “Early steps in steroidogenesis: intracellular cholesterol trafficking”. Journal of Lipid Research. 52 (12): 2111–2135. doi:10.1194/jlr.R016675. PMC 3283258. PMID 21976778. Charmandari, E; Brook, CG; Hindmarsh, PC (2004). “Classic congenital adrenal hyperplasia and puberty”. European Journal of Endocrinology. 151 (Suppl 3): 77–82. CiteSeerX 10.1.1.613.6853. doi:10.1530/eje.0.151U077. PMID 15554890. Archived from the original on 4 February 2015. Crowley, SD; Coffman, TM (2012). “Recent advances involving the renin–angiotensin system”. Experimental Cell Research. 318 (9): 1049–1056. doi:10.1016/j.yexcr.2012.02.023. PMC 3625040. PMID 22410251. Hall JE, Guyton AC (2010). Guyton and Hall Textbook of Medical Physiology, 12th edition. Saunders. ISBN 978-1416045748. Henry Gleitman, Alan J. Fridlund and Daniel Reisberg (2004). Psychology (6 ed.). W. W. Norton & Company. ISBN 978-0-393-97767-7. García, AG; García de Diego, AM; Gandía, L; Borges, R; García Sancho, J (2006). “Calcium signaling and exocytosis in adrenal chromaffin cells”. Physiological Reviews. 86 (4): 1093–1131. doi:10.1152/physrev.00039.2005. PMID 17015485. “The human proteome in adrenal gland – The Human Protein Atlas”. [url]www.proteinatlas.org.[/url] Retrieved 21 September 2017. Uhlén, Mathias; Fagerberg, Linn; Hallstrm, Bjrn M.; Lindskog, Cecilia; Oksvold, Per; Mardinoglu, Adil; Sivertsson, sa; Kampf, Caroline; Sjstedt, Evelina (23 January 2015). “Tissue-based map of the human proteome”. Science. 347 (6220): 1260419. doi:10.1126/science.1260419. ISSN 0036-8075. PMID 25613900. S2CID 802377. Bergman, Julia; Botling, Johan; Fagerberg, Linn; Hallstrm, Bjrn M.; Djureinovic, Dijana; Uhlén, Mathias; Pontén, Fredrik (1 February 2017). “The Human Adrenal Gland Proteome Defined by Transcriptomics and Antibody-Based Profiling”. Endocrinology. 158 (2): 239–251. doi:10.1210/en.2016-1758. ISSN 0013-7227. PMID 27901589. Barwick, T.D.; Malhotra, A.; Webb, J.A.W.; Savage, M.O.; Reznek, R.H. (September 2005). “Embryology of the adrenal glands and its relevance to diagnostic imaging”. Clinical Radiology. 60 (9): 953–959. doi:10.1016/j.crad.2005.04.006. PMID 16124976. Ishimoto H, Jaffe RB (2011). “Development and Function of the Human Fetal Adrenal Cortex: A Key Component in the Feto-Placental Unit”. Endocrine Reviews. 32 (3): 317–355. doi:10.1210/er.2010-0001. PMC 3365797. PMID 21051591. Hoeflich A, Bielohuby M (2009). “Mechanisms of adrenal gland growth: signal integration by extracellular signal regulated kinases1/2”. Journal of Molecular Endocrinology. 42 (3): 191–203. doi:10.1677/JME-08-0160. PMID 19052254. Mesiano S, Jaffe RB (1997). “Developmental and Functional Biology of the Primate Fetal Adrenal Cortex”. Endocrine Reviews. 18 (3): 378–403. doi:10.1210/edrv.18.3.0304. PMID 9183569. Hornsby, PJ (2012). “Adrenarche: a cell biological perspective”. The Journal of Endocrinology. 214 (2): 113–119. doi:10.1530/JOE-12-0022. PMID 22573830. Rege, J; Rainey, WE (2012). “The steroid metabolome of adrenarche”. The Journal of Endocrinology. 214 (2): 133–143. doi:10.1530/JOE-12-0183. PMC 4041616. PMID 22715193. Huber K (2006). “The sympathoadrenal cell lineage: Specification, diversification, and new perspectives”. Developmental Biology. 298 (2): 335–343. doi:10.1016/j.ydbio.2006.07.010. PMID 16928368. Unsicker K, Huber K, Schober A, Kalcheim C (2013). “Resolved and open issues in chromaffin cell development”. Mechanisms of Development. 130 (6–8): 324–329. doi:10.1016/j.mod.2012.11.004. PMID 23220335. Hydrocortisone Emergency Factsheet for Ambulance Personnel The Pituitary Foundation Data and references for pie chart are located at file description page in Wikimedia Commons. Mantero, F; Terzolo, M; Arnaldi, G; Osella, G; Masini, AM; Alì, A; Giovagnetti, M; Opocher, G; Angeli, A (2000). “A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology”. The Journal of Clinical Endocrinology and Metabolism. 85 (2): 637–644. doi:10.1210/jcem.85.2.6372. PMID 10690869. Martucci, VL; Pacak, K (2014). “Pheochromocytoma and paraganglioma: diagnosis, genetics, management, and treatment”. Current Problems in Cancer. 38 (1): 7–41. doi:10.1016/j.currproblcancer.2014.01.001. PMC 3992879. PMID 24636754. Schmidt, JE (1959). Medical Discoveries: Who and When. Thomas. pp. 9–10. O’Hare, A. Munro Neville, Michael J. (2012). The Human Adrenal Cortex Pathology and Biology – An Integrated Approach. London: Springer London. pp. Chapter 2: Historical Aspects. ISBN 978-1447113171. “What Are The Adrenal Glands?”. About.com. Retrieved 18 September 2013. Pearce, JM (2004). “Thomas Addison (1793–1860)”. Journal of the Royal Society of Medicine. 97 (6): 297–300. doi:10.1258/jrsm.97.6.297. PMC 1079500. PMID 15173338. “The Nobel Prize in Physiology or Medicine 1950”. Nobel Foundation. Retrieved 10 February 2015.
All sharing and opinions are for professional communication and reference only
References and images are sourced from the internet, copyright belongs to the original authors
WeChat public account “Ding Xiang Ye” ID: dxyeyx