
|
|
Bletilla, also known as Bletilla striata (Thunb.) Reichb.f., is a dried tuber of the orchid plant, widely distributed in various provinces and cities in China, and grows in moist places such as valleys and mountains. Bletilla striata polysaccharide (BSP) is the main active component of Bletilla, which has attracted attention due to its significant effects on immune regulation, antioxidant, anticancer, hemostatic, anti-inflammatory, antibacterial, gastric protection, and liver protection. The liver maintains many important functions in the body, including detoxification, metabolism, protein synthesis, host defense, and immune response. Studies have shown that lipopolysaccharide (LPS) binds to corresponding proteins upon entering the liver, connecting with Toll-like receptor 4 (TLR4), stimulating the MyD88-dependent pathway mediated by TLR4, thereby activating the NF-κB pathway, inducing lipid peroxidation and producing pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNFα), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β). He Guofang et al. found that BSP has a protective effect on acute alcoholic liver injury in mice, significantly reducing alanine aminotransferase (ALT) activity in liver tissue and increasing superoxide dismutase (SOD) and glutathione (GSH) levels, improving liver cell swelling and degeneration. Luo et al. studied the effect of BSP on the epithelial barrier destruction in rats induced by thioacetamide, finding that BSP could reduce endotoxin levels and inhibit inflammatory cytokines IL-6 and TNF-α. Other studies have shown that BSP can treat non-alcoholic fatty liver by reducing lipid accumulation and fibrosis in liver tissue. To better develop and utilize BSP, this study explores the protective effect and mechanism of BSP on LPS-induced acute liver injury, aiming to provide experimental evidence for the development and utilization of Bletilla.
1 Materials and Methods
1.1 Reagents and Instruments
Sixty healthy male Kunming mice, weighing (18±2)g, were purchased from Liaoning Changsheng Biotechnology Co., Ltd., Animal License No: SCXK(Liao)2020-0001.
BSP (Shaanxi Zelang Biotechnology Co., Ltd., Batch No: ZLSW221117-1, purity ≥98%); biphenyl ester drops (BDP, Wanbond Pharmaceutical Group Co., Ltd., Batch No: 19J211148); LPS (Solarbio, Batch No: 325D031); ALT, aspartate aminotransferase (AST), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), SOD, catalase (CAT) kits (Nanjing Jiancheng Bioengineering Institute); TLR4, MyD88, NF-κB, TNF-α, IL-1β, IL-6, β-actin, HRP-labeled goat anti-rabbit IgG antibody (Wuhan Aibotai Biological Technology Co., Ltd.).
SPARK type enzyme marker (Switzerland Tecan); electrophoresis apparatus, transfer tank, immunoblotting workstation (USA BIO-RAD); HI1210 embedding machine, HI1220 baking machine, HistoCore tissue dehydrator, HistoCore Arcadia C paraffin embedding machine, HistoCore AUTOCUT slicer, DM2000 upright optical microscope (Leica); low-temperature high-speed centrifuge TGL-20M (Hunan Xiangyi Centrifuge Instrument Co., Ltd.).
1.2 Methods
Sixty male Kunming mice were housed in a standard animal room [temperature 22℃~25℃, humidity (50±5)%], after 7 days of adaptive feeding, randomly divided into 6 groups, with 10 mice in each group: blank group (Control group), model group (LPS 1mg/kg, Model group), positive control group (2mg/kg, BDP group), low-dose group (BSP 100mg/kg group), medium-dose group (BSP 200mg/kg group), high-dose group (BSP 400mg/kg group). Except for the Control and Model groups, the other 4 groups were administered corresponding drug doses, 1 time/d, for 7 consecutive days, and 2 hours after the last administration, an intraperitoneal injection of LPS (1mg/kg) was given to establish the acute liver injury model. The Control group was given an equal volume of saline and fasted, with free access to drinking water. Blood was collected from the eyeball 12 hours after LPS administration, and the mice were sacrificed to obtain liver tissue.
The liver tissue of mice was soaked in 10% neutral formaldehyde solution, fixed for 24 hours, dehydrated, cleared, embedded in paraffin, sliced, stained with HE, and resin sealed for observation under an optical microscope.
The collected blood was centrifuged to separate serum (4℃, 4000r/min, 10min) to obtain the supernatant, and ALT, AST, SOD, MDA, GSH-PX, and CAT levels were determined according to the kit instructions.
The liver tissue was cut into pieces weighing 30mg, lysed, centrifuged, and the supernatant was extracted. The concentration was determined by the BCA method, denatured at 95℃, and then added to the 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample wells for constant pressure electrophoresis; at a constant current of 220mA for 70 minutes, the proteins were transferred to a PVDF membrane, blocked with skim milk on a shaker for 60 minutes, washed with TBST, and then the membrane was incubated overnight at 4℃ with diluted primary antibodies for TLR4, MyD88, NF-κB, TNF-α, IL-1β, IL-6, and internal reference β-actin (dilution ratios of 1:1000, 1:1000, 1:1000, 1:1000, 1:1000, 1:1000, 1:5000). The next day, after washing the membrane three times with TBST, HRP-labeled goat anti-rabbit IgG antibody (dilution of 1:10000) was added and incubated at room temperature for 60 minutes; after washing three times with TBST, ECL development was performed for imaging. Image Lab software was used for grayscale analysis of the images, and the relative expression change was represented by the ratio of the grayscale values of the target protein to the internal reference protein, indirectly reflecting the protein expression level.
1.3 Statistical Methods
Data were expressed as 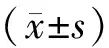 indicates normality test, analyzed using Graphpad Prism 8.0 software, one-way ANOVA, and set P<0.05 to indicate statistical significance.
indicates normality test, analyzed using Graphpad Prism 8.0 software, one-way ANOVA, and set P<0.05 to indicate statistical significance.
2 Results
2.1 Changes in Body Weight and Liver Index of Mice
From the results of body weight changes, the average body weight of each group of mice showed an increasing trend, indicating that the overall health status of the mice was good during the administration period, with no significant weight loss or growth retardation; at the same time, it was observed that the fur gloss of mice in each group was normal, indicating that the mice had normal feeding and nutrient absorption; in addition, there were no significant differences in the behavior and activity of mice in each group, indicating that the drugs did not negatively affect the daily behavior of the mice. Compared with the Control group, the liver index in the Model group significantly increased (P<0.01), suggesting that the liver may be enlarged; compared with the Model group, the liver index in the BDP group and the BSP 200, 400mg/kg groups significantly decreased (P<0.01). See Table 1.
Table 1 Effect of BSP Intervention on Body Weight and Liver Index of Mice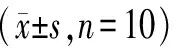
Compared with the Control group, **P<0.01; compared with the Model group, ##P<0.01.
2.2 Morphological Changes of Liver Tissue and Pathological Changes of Liver
The liver of the Control group appeared reddish-brown, with a smooth surface, soft texture, and normal shape and size; in the Model group, the liver showed punctate hemorrhage and varying degrees of yellowing, with surface and edge damage; the liver appearance in the BDP and each BSP administration group was smoother, with reduced damage, occasional bleeding points and abnormal changes. See Figure 1A.
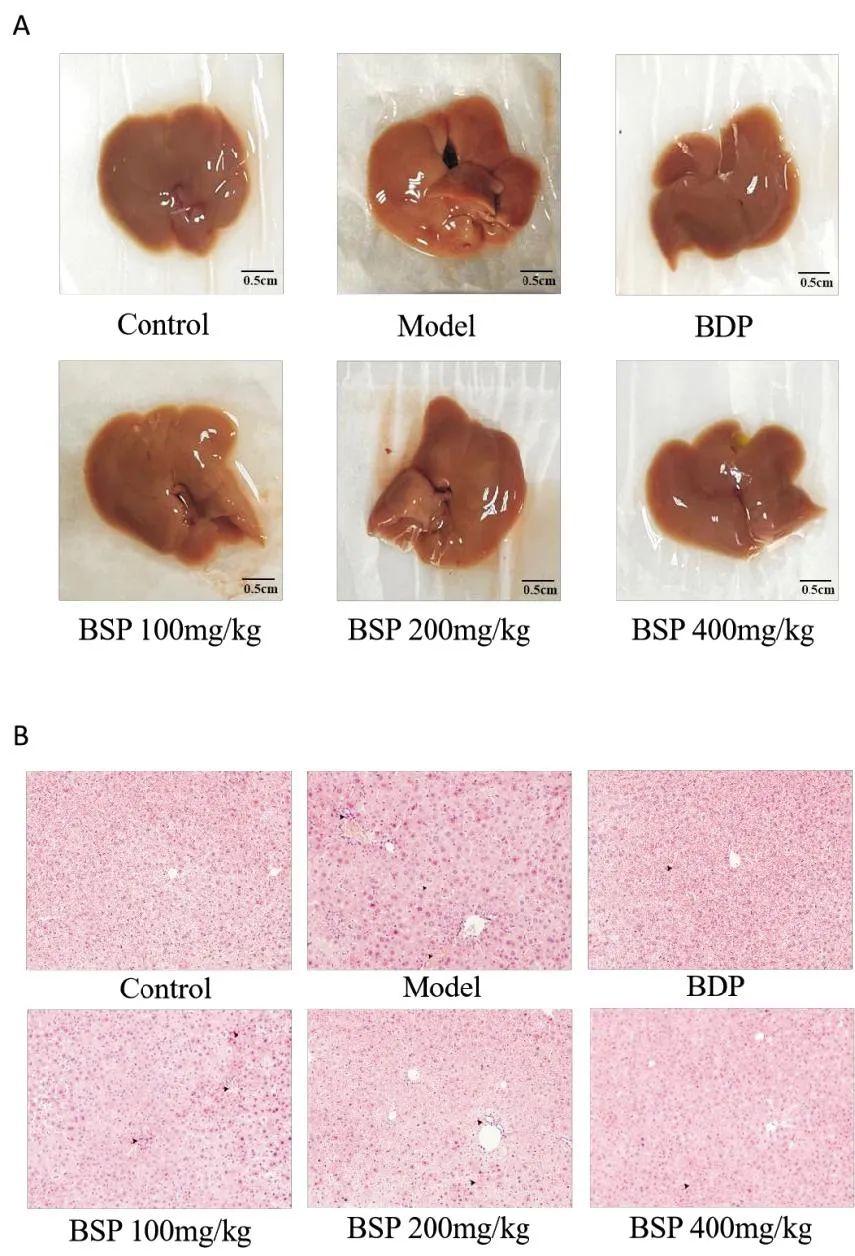
A. Morphological Changes of Mouse Liver Tissue; B. Pathological Changes of Liver Tissue (100×); ▲: Lymphocyte and Macrophage Aggregation.
Figure 1 Morphological Changes of Mouse Liver Tissue and Pathological Changes of Liver Tissue
As shown in Figure 1B, the liver lobule structure of the Control group was normal, with orderly cell arrangement, clear structure, and no inflammatory cell infiltration near the central vein, with no pathological changes such as cell necrosis or degeneration. The liver tissue of the Model group showed significant pathological changes, including unclear liver lobule structure, disordered arrangement of liver cells, a large number of inflammatory cell infiltrations, and obvious vacuolation. In contrast, compared with the Model group, the liver tissue of the BDP and BSP administration groups showed significant improvement in pathological changes, with reduced macrophage infiltration, alleviated pathological changes, and particularly significant improvement in the BSP 400mg/kg group, with a relatively intact liver lobule structure and significantly reduced cellular vacuolation.
2.3 Effect of BSP on Plasma ALT and AST in Mice with LPS-Induced Acute Liver Injury
As shown in Table 2, compared with the Control group, the ALT and AST levels in the Model group significantly increased (P<0.01), indicating that LPS induced significant liver injury in mice; compared with the Model group, the BDP group and BSP administration groups significantly reduced the elevated plasma AST and ALT activities caused by LPS (P<0.01).
Table 2 ALT and AST Levels of Mice in Each Group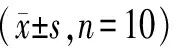
Compared with the Control group, **P<0.01; compared with the Model group, ##P<0.01.
2.4 Effect of BSP on Plasma SOD, MDA, GSH-PX, and CAT in Mice with LPS-Induced Acute Liver Injury
Compared with the Control group, the MDA level in the Model group significantly increased, while SOD, GSH-PX, and CAT levels significantly decreased (P all <0.01); compared with the Model group, the MDA level significantly decreased in the BDP group and BSP administration groups, while CAT significantly increased (P all <0.01), and SOD and GSH-PX levels significantly increased in the BDP group and BSP 200mg/kg and 400mg/kg groups (P all <0.01), with statistical significance. See Table 3.
Table 3 Levels of SOD, MDA, GSH-PX, and CAT in Mice of Each Group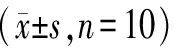
Compared with the Control group, **P<0.01; compared with the Model group, ##P<0.01.
2.5 Effect of BSP on TLR4/MyD88/NF-κB Signaling Pathway Protein Expression
As shown in Figure 2, compared with the Control group, the protein expression levels of TLR4, MyD88, and NF-κB in the Model group significantly increased (P<0.01); compared with the Model group, the expression of TLR4 protein decreased in the BSP 100mg/kg group (P<0.05), the expression of NF-κB protein decreased in the BSP 200mg/kg group (P<0.05), and the expression of TLR4 and MyD88 proteins significantly decreased (P<0.01). In the BDP group and BSP 400mg/kg group, the expression levels of all three proteins significantly decreased (P<0.01).
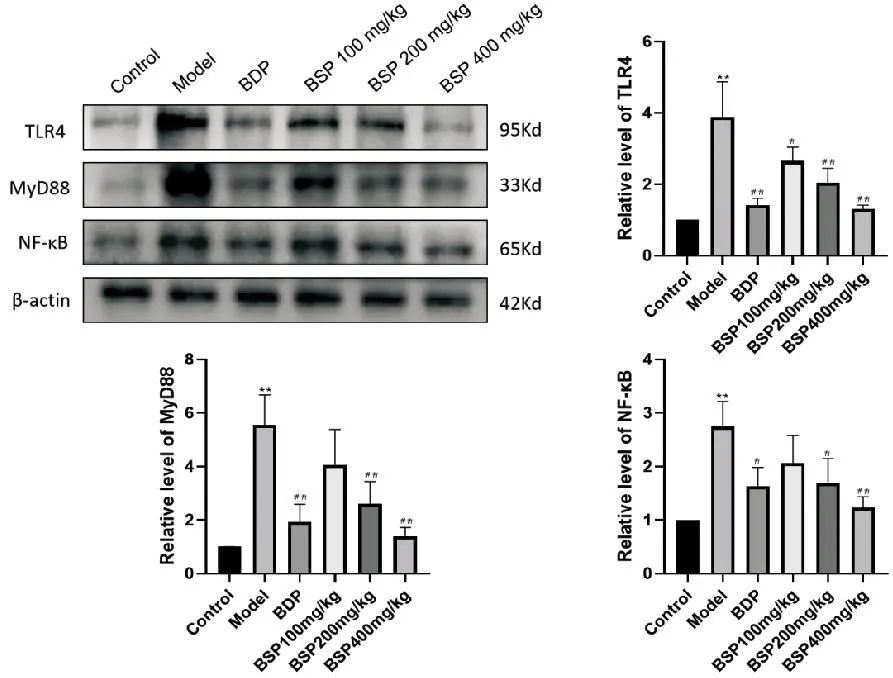
Compared with the Control group, **P<0.01; compared with the Model group, #P<0.05, ##P<0.01.
Figure 2 Effect of BSP Intervention on Related Pathway Protein Levels (n=3)
2.6 Effect of BSP on TNF-α/IL-6/IL-1β Inflammatory-Related Protein Expression
As shown in Figure 3, compared with the Control group, the protein expression levels of TNF-α, IL-6, and IL-1β in the Model group significantly increased (P<0.01); compared with the Model group, the expression of IL-6 protein decreased in the BSP 200mg/kg group (P<0.05), and the expression of TNF-α protein significantly decreased (P<0.01). In the BDP group and BSP 400mg/kg group, the expression levels of all three proteins significantly decreased (P<0.01).
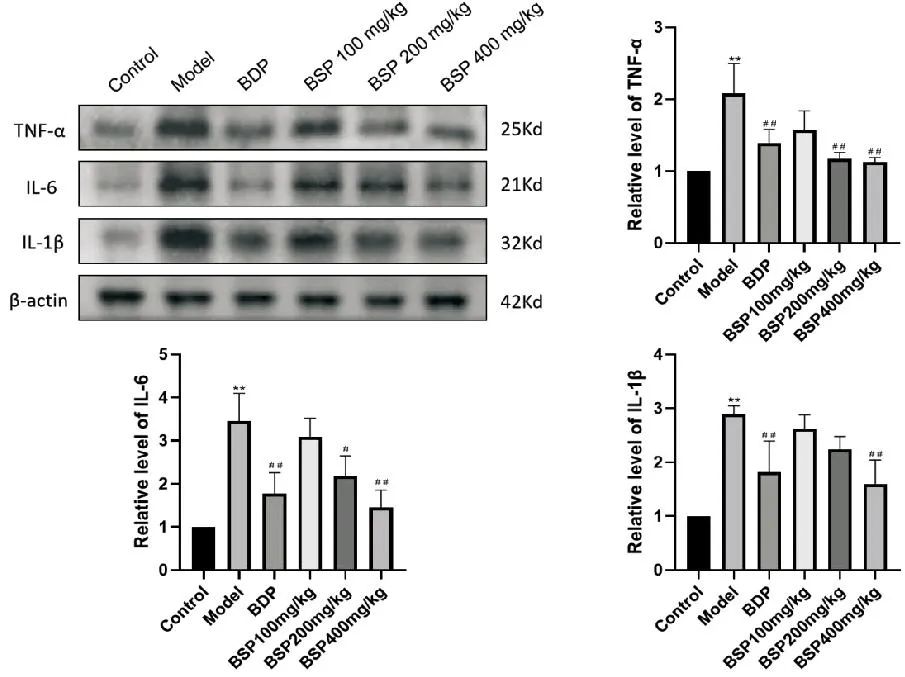
Compared with the Control group, **P<0.01; compared with the Model group, #P<0.05, ##P<0.01.
Figure 3 Effect of BSP Intervention on Inflammatory-Related Protein Levels (n=3)
3 Discussion
The liver is an important metabolic organ in the body, and inflammation is one of the common causes of liver injury, caused by various pathogenic factors such as viruses, drugs, alcohol, and autoimmune factors. LPS is an effective stimulant of the immune system, and as the concentration of LPS increases, the expression of inflammatory mediators in the body increases, inducing inflammatory damage to organs. TLR4 is the main signaling receptor for LPS and is closely related to the pathogenesis and treatment of liver function damage. MyD88 is the key downstream signaling protein interacting with TLR4 and LPS, and under the action of LPS, NF-κB is activated through the TLR4-MyD88 signaling pathway, ultimately leading to the activation of inflammatory responses and the release of inflammatory factors. At the same time, almost all liver diseases are accompanied by varying degrees of oxidative stress, so effectively inhibiting oxidative stress is also an important means of liver protection. CAT is the endogenous antioxidant enzyme with the highest capacity to remove reactive oxygen species in liver cells and is a sensitive marker of tissue oxidative stress levels. MDA is the final product of lipid peroxidation processes in the body, and excessive MDA can exacerbate liver cell damage and necrosis, promoting the release of inflammatory factors such as TNF-α, IL-6, and IL-1β, which in turn promote oxidative stress, further aggravating liver injury. At the same time, SOD and GSH-Px present in the body can protect the body from oxidative stress damage caused by free radicals and reactive oxygen species. Therefore, regulating inflammatory signaling pathways to inhibit inflammation and exert antioxidant effects may be an effective method to prevent LPS-induced acute liver injury.
In this study, by measuring the plasma levels of liver function-related transaminases AST and ALT in mice, observing tissue pathological changes, calculating liver index, and other aspects to evaluate liver status, it was found that the plasma AST and ALT levels in mice induced by LPS significantly increased, with a large number of inflammatory cell infiltrations, disordered liver cords, and unclear liver structure, and the liver index of mice significantly increased. All these changes directly indicate that the intraperitoneal injection of LPS to establish an acute liver injury model is successful, and the administration groups can significantly improve these conditions. By detecting oxidative stress-related indicators, the MDA level in the Model group significantly increased compared to the Control group, while the activities of SOD, GSH-Px, and CAT significantly decreased; however, both the medium and high doses of BSP could significantly reduce MDA content and increase SOD, GSH-Px, and CAT activities, suggesting that medium and high doses of BSP can effectively reduce the oxidative stress level in mice with LPS-induced acute liver injury. The Western blot results showed that the intervention of BSP could reduce the expression of TLR4/MyD88/NF-κB in liver tissue induced by LPS, and the expression of inflammatory-related proteins TNF-α, IL-6, and IL-1β also decreased. Therefore, BSP has a certain protective effect on LPS-induced acute liver injury in mice, and its mechanism may be related to antioxidant effects and inhibition of the TLR4/MyD88/NF-κB signaling pathway. The results of this study provide a theoretical basis for further development and application of BSP, serving as a reference for BSP as an effective candidate drug for LPS-induced liver injury.
