
Serous ovarian cancer (serous ovarian cancer, SOC) has a poor prognosis due to the high invasiveness and cisplatin resistance of SOC cells, while the molecular mechanisms remain largely unknown.Snail is a classic biomarker of EMT and plays a crucial role in cancer cell transformation, progression, metastasis, and chemoresistance. This study identified the expression and function of the gene MYH10, which encodes non-muscle myosin heavy chain IIB, in SOC through immunohistochemistry, in vitro, and in vivo studies.The mechanism of MYH10 was confirmed through co-immunoprecipitation, GST pull-down, and confocal laser microscopy. The results indicated that knockdown of MYH10 inhibited the proliferation, migration, invasion, metastasis, and cisplatin resistance of SOC cells both in vitro and in vivo. Further studies confirmed that the functional domain of MYH10 protein interacts with the gene MYH9, which encodes non-muscle myosin heavy chain IIA, recruiting deubiquitinating enzyme USP45 to deubiquitinate snail, inhibiting its protein degradation, ultimately promoting tumorigenesis, progression, and cisplatin resistance in SOC. In clinical samples, the expression of MYH10 was significantly increased in SOC samples compared to paraneoplastic samples.The expression of MYH10 positively correlated with that of MYH9. Co-expression of MYH10+/MYH9+ is an independent prognostic factor predicting survival in SOC patients. These findings reveal the critical role of the MYH10-MYH9-Snail axis in the carcinogenesis, progression, and cisplatin resistance of SOC, providing potential new therapeutic targets for SOC intervention. This article was published in Advanced Science (Weinheim, Baden-Wurttemberg, Germany) in May 2023.
Technical Route:
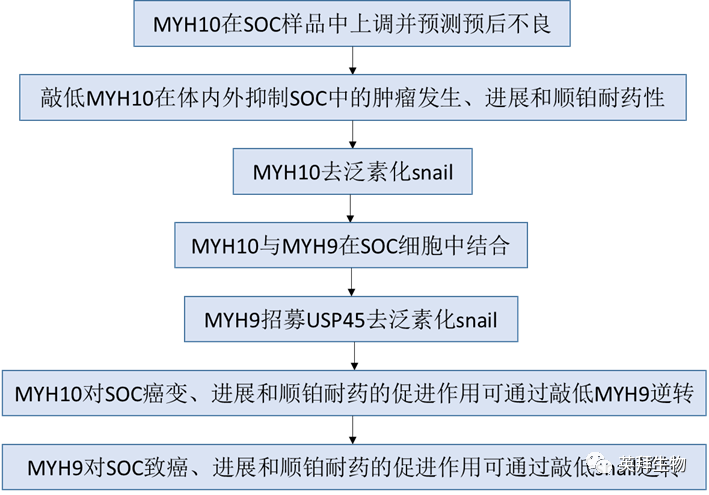
Main Experimental Results:
1, MYH10 is upregulated in SOC samples and predicts poor prognosis
To explore the clinical significance of MYH10 in SOC samples, this study performed IHC to detect the expression of MYH10 protein (Figure 1B, C), and the results showed that the expression of MYH10 protein was upregulated in SOC samples compared to paraneoplastic samples (Figure 1A). Importantly, Kaplan-Meier analysis indicated that high expression of MYH10 correlated with shorter overall survival (OS) and recurrence-free survival (RFS) in SOC samples (Figure 1D). In addition, the expression of MYH10 was associated with FIGO staging, peritoneal metastasis, and intestinal metastasis (Table 1). These results indicate that MYH10 is upregulated in SOC and can predict poor prognosis.
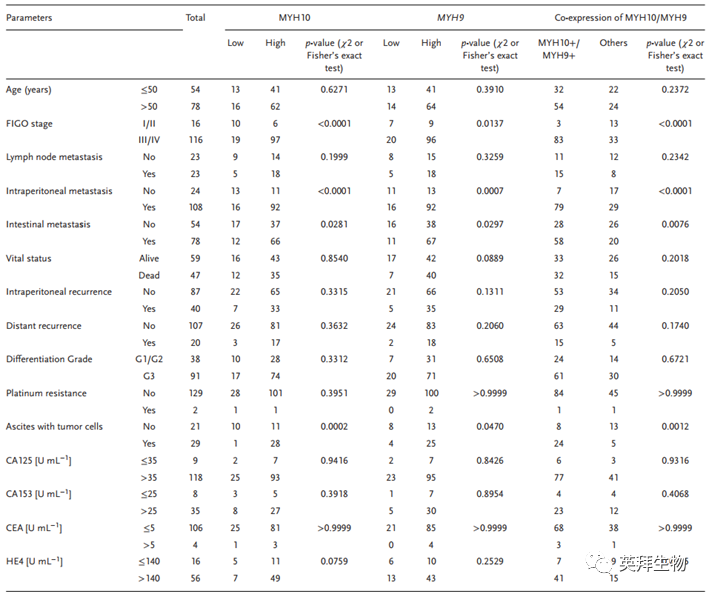
Figure 1 Upregulation of MYH10 predicts poor prognosis in SOC patients
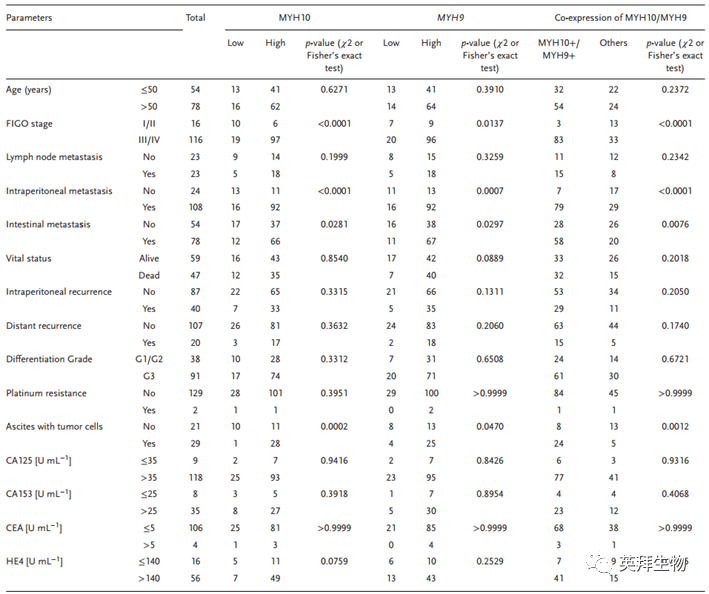
Table 1 Correlation of MYH10, MYH9, or MYH10/MYH9 expression with standard clinical pathological variables using χ2 or Fisher’s exact test
2, Knockdown of MYH10 inhibits tumorigenesis, progression, and cisplatin resistance in SOC both in vitro and in vivo
To verify the oncogenic function of MYH10, this study used different siRNA to effectively knock down MYH10 expression in SOC cells. Using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), Edu, and colony formation assays (Figure 2A-C), knockdown of MYH10 inhibited the growth and proliferation of SOC cells. Furthermore, Transwell, Boyden, and scratch assays indicated that knockdown of MYH10 could inhibit the migration and invasion of SOC cells (Figure 2D, E), and in vitro drug sensitivity tests showed that the IC50 value of MYH10 knockdown cells was statistically lower than that of the control (Figure 2G), and this effect was further confirmed in the shMYH10 nude mouse xenograft model (Figure 2F).Kaplan-Meier method estimated the survival time, confirming that compared to untreated normal controls (NC+NS), single cisplatin treatment (NC+cisplatin) or shMYH10 (shMYH10+ saline [NS]) effectively prolonged survival time. However, the shMYH10+cisplatin group significantly extended survival time, exceeding that of the other three groups (Figure 2F).The median survival time of the NC+NS group was 20 days, shMYH10+NS group was 31.5 days, the cisplatin+NS group was 38.5 days, and the shMYH10+cisplatin group was 47.5 days. Additionally, to explore whether MYH10 affects tumor growth and lung metastasis, subcutaneous and tail vein injection graft tumor experiments were conducted in nude mice, and the results showed that knockdown of MYH10 inhibited in vivo tumor proliferation (Figure 2H) and metastasis (Figure 2I), with mice injected with shMYH10 cells showing smaller tumor size and weight compared to controls. Furthermore, Western blot analysis showed that EMT signals including N-cadherin, vimentin, snail, and slug were significantly inhibited, while E-cadherin was significantly upregulated (Figure 2J). These results indicate that the knockdown of the MYH10 gene inhibits the proliferation, migration, invasion, metastasis, and cisplatin resistance of SOC through the EMT signaling pathway both in vitro and in vivo. These data suggest that MYH10 acts as an oncogene, promoting the carcinogenesis, progression, and cisplatin resistance of SOC in vitro and in vivo.
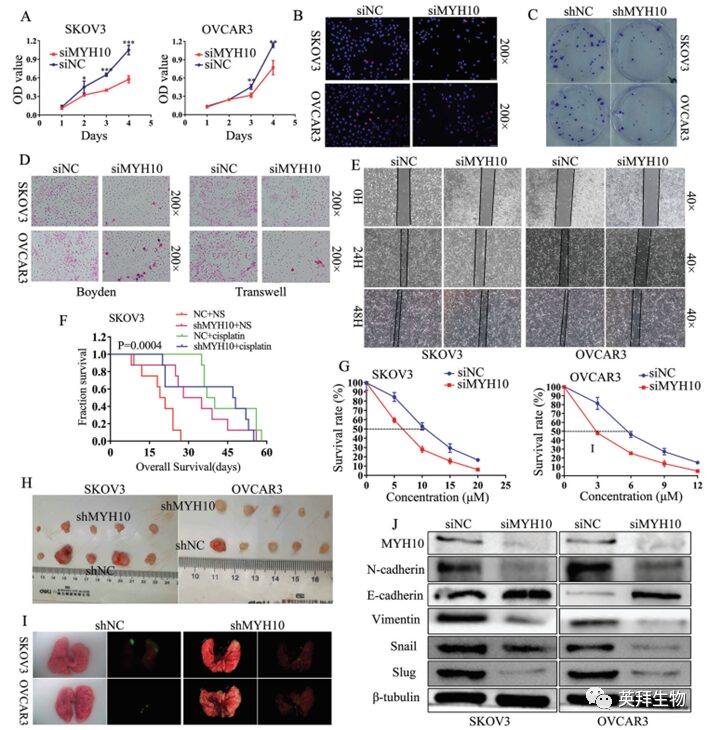
Figure 2 Knockdown of MYH10 inhibits the proliferation, migration, invasion, metastasis, and cisplatin resistance of SOC cells through the inactivation of EMT signaling
3, MYH10 deubiquitinates snail
To further determine the mechanism of MYH10 in SOC cells, the study used the Biogrid bioinformatics website to predict candidate interacting proteins and found that snail is a candidate interacting protein of MYH10. Additionally, the authors used RT-qPCR and WB to detect the expression of snail after knockdown of MYH10, finding that the mRNA expression of snail did not change (Figure 3A), while protein expression was downregulated after knockdown of MYH10 (Figure 2L). Furthermore, endogenous Co-IP assays observed that MYH10 interacts with snail (Figure 3B), and it was further verified whether MYH10 reduces the degradation of snail by inhibiting its ubiquitination in SOC cells. Specific anti-snail antibodies were used to immunoprecipitate snail, and anti-ubiquitin antibodies were used to analyze its ubiquitination status. As expected, overexpression of MYH10 significantly reduced the ubiquitination level of snail (Figure 3C). In addition, confocal laser microscopy confirmed that MYH10 and snail co-localize in the cytoplasm of SOC cells (Figure 3D), and knockdown of MYH10 could impair the stability of snail in SOC cells treated with actinomycin D and MG132 at different time points (Figure 3E, F).
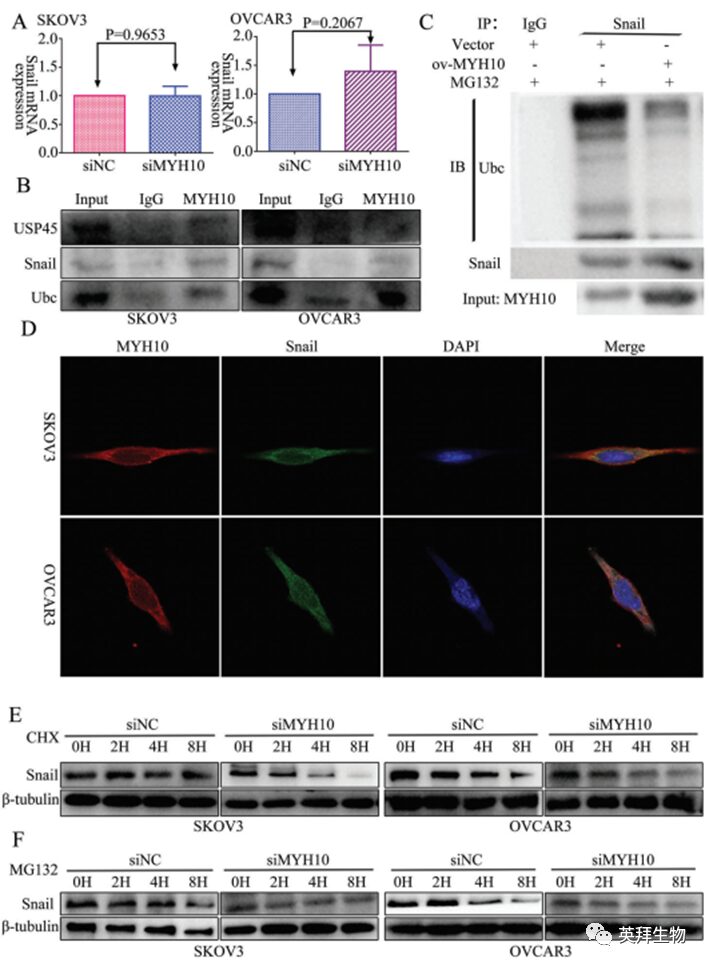
Figure 3 MYH10 deubiquitinates snail protein
4, MYH10 interacts with MYH9 in SOC cells
To further verify the detailed mechanism of MYH10 in SOC cells, the authors again used Biogrid to predict candidate interacting proteins and found that MYH9 is a candidate interacting protein of MYH10. In previous studies, it has been found that MYH9 is upregulated in OC tissues and promotes the proliferation, migration, invasion, and metastasis of SOC by regulating the Wnt/β-catenin pathway and EMT signaling. Furthermore, the authors used both exogenous and endogenous Co-IP assays to show that the functional domain of MYH10 (≈879-1959aa) interacts with MYH9 (Figure 4C-F), and confocal laser microscopy confirmed that MYH10 and MYH9 co-localize in the cytoplasm of SOC cells (Figure 4G). Additionally, GST pull-down assays indicated that in SOC cell lines, MYH10 can directly bind to MYH9 (Figure 4D). To determine the relationship between the expression of MYH10 and MYH9, the study performed IHC on a total of 132 pairs of SOC samples (Figure 4A). Kaplan-Meier analysis clearly showed that MYH10+/MYH9+ patients had shorter OS or RFS compared to other expressions (Figure 4B). The expression of MYH10+/MYH9+ was directly related to FIGO staging, peritoneal metastasis, intestinal metastasis, ascitic tumor cells, and serum HE4 levels. Moreover, co-expression of MYH10+/MYH9+ is indeed an independent prognostic factor, rather than using MYH10 or MYH9 alone in multivariate analysis (Table 2). Notably, the positive correlation between the protein levels of MYH10 and MYH9 was detected in SOC samples (Table 3).
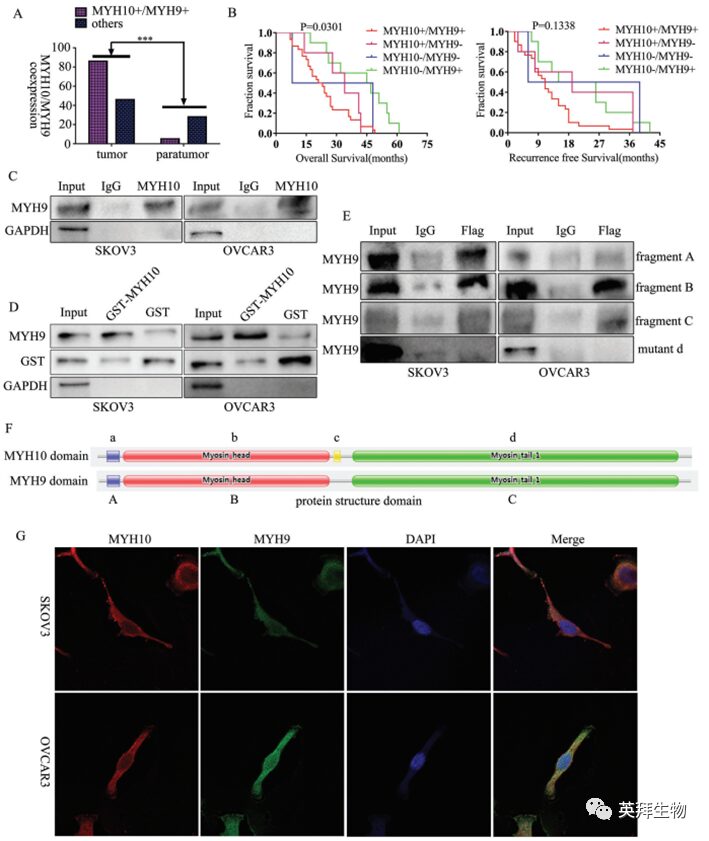
Figure 4 MYH10 interacts with MYH9 in SOC cells
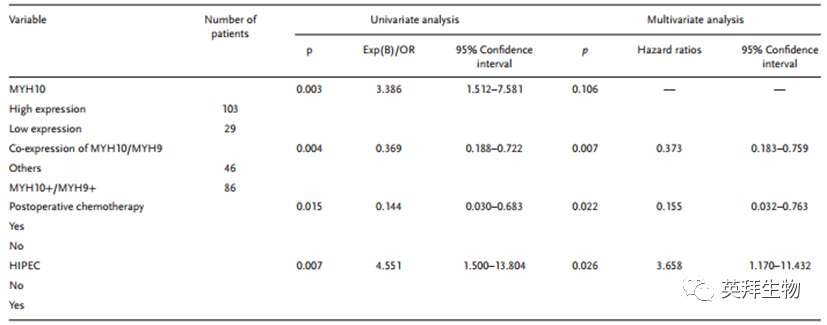
Table 2 Univariate and multivariate Cox regression analysis of prognostic factors in SOC
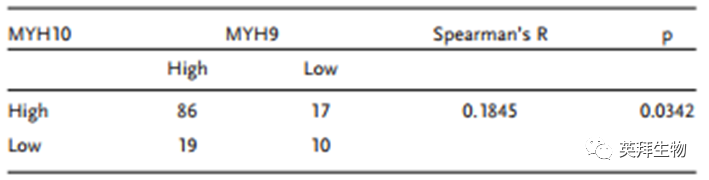
Table 3 Correlation study between MYH10 and MYH9 expression
5, MYH9 recruits USP45 to deubiquitinate snail
In previous studies, it is known that MYH9 promotes the proliferation, migration, invasion, and metastasis of OC by regulating the Wnt/β-catenin pathway and EMT signaling. To explore the influence of MYH9 on snail, the authors used RT-qPCR and WB to detect the expression of snail after knockdown of MYH9, and the results showed that the mRNA expression of snail did not change (Figure 5A), while the protein expression of snail was downregulated (Figure 5B). To further verify the role of MYH9 in SOC cells, the authors applied the Biogrid bioinformatics website to predict candidate interacting proteins and found that USP45 and snail are candidate interacting proteins of MYH9. Additionally, endogenous Co-IP analysis showed that MYH9 can interact with USP45, snail, and ubiquitin (Figure 5C), and Co-IP assays indicated that knockdown of USP45 could improve the effects of MYH9 on the deubiquitination and stability of snail (Figure 5D). Furthermore, confocal laser microscopy confirmed that both MYH9 and USP45 as well as MYH9 and snail co-localize in the cytoplasm of SOC cells (Figure 5G, H), and knockdown or overexpression of MYH9 may impair the stability of snail at different time points in SOC cells treated with actinomycin D and MG132 (Figure 5E, F). Additionally, the study found that knockdown of USP45 could also impair the stability of snail at different time points in SOC cells treated with actinomycin D and MG132 (Figure 5I, J).

Figure 5 MYH9 recruits USP45 to deubiquitinate snail
6, The promoting effect of MYH10 on SOC carcinogenesis, progression, and cisplatin resistance can be reversed by knocking down MYH9
To determine whether knockdown of MYH9 reverses the promoting effect of MYH10 on SOC carcinogenesis, progression, and cisplatin resistance, MTT and EdU assays were conducted to verify the effect of siMYH9 on the proliferation of MYH10 overexpressing SOC cells (Figure 6A, C). Additionally, Transwell and scratch assays were performed to explore the reversing effect of siMYH9 on the migration and invasion of MYH10 overexpressing SOC cells (Figure 6D, E). Moreover, the IC50 value was detected to verify the effect of siMYH9 on MYH10 overexpressing SOC cells (Figure 6B), and WB was performed to explore the potential mechanisms. WB showed that knockdown of MYH9 could reverse the promotion of MYH10 on the EMT signals (Figure 6F). These data indicate that the promoting effect of MYH10 on SOC carcinogenesis, progression, and cisplatin resistance can be reversed by knocking down MYH9.

Figure 6 Knockdown of MYH9 reverses the MYH10 induced SOC carcinogenesis, progression, and cisplatin resistance
7, The promoting effect of MYH9 on SOC carcinogenesis, progression, and cisplatin resistance can be reversed by knocking down snail
To determine whether knockdown of snail reverses the promoting effect of MYH9 on SOC carcinogenesis, progression, and cisplatin resistance, MTT and EdU assays were conducted to verify the effect of si-snail on the proliferation of MYH9 overexpressing SOC cells (Figure 7A, C). Additionally, Transwell and scratch assays were performed to explore the reversing effect of si-snail on the migration and invasion of MYH9 overexpressing SOC cells (Figure 7D, E). Moreover, the IC50 value was detected to verify the effect of si-snail on MYH9 overexpressing SOC cells (Figure 7B), and WB was performed to explore its potential mechanisms, and WB showed that knockdown of snail could reverse the promotion of MYH9 on the EMT signals (Figure 7F). These data indicate that the promoting effect of MYH9 on SOC carcinogenesis, progression, and cisplatin resistance can be reversed by knocking down snail.

Figure 7 Knockdown of snail reverses the promoting effect of MYH9 induced SOC carcinogenesis, progression, and cisplatin resistance

Figure 8 Model diagram of this study
In summary, this study indicates that MYH10 is upregulated in SOC samples and predicts poor prognosis for SOC patients.MYH10 acts as an oncogene in SOC, promoting cisplatin resistance.MYH9 is an interacting protein of MYH10, which recruits USP45 to deubiquitinate snail, leading to the activation of the EMT signaling pathway, thereby promoting the carcinogenesis, progression, and cisplatin resistance of SOC (Figure 8). This study provides new insights into the molecular mechanisms associated with the occurrence, progression, and cisplatin resistance of SOC and potential biomarkers for its treatment.
References:
Liu L, Chen C, Liu P, et al. MYH10 Combines with MYH9 to Recruit USP45 by Deubiquitinating Snail and Promotes Serous Ovarian Cancer Carcinogenesis, Progression, and Cisplatin Resistance. Adv Sci (Weinh). 2023; 10 (14): e2203423. doi:10.1002/advs.202203423

Routine Molecular Experiments: qRT-PCR, WB, Co-IP, GST pull-down, confocal laser analysis, immunohistochemistry (IHC) staining
Cell Experiments: Transfection, MTT Assay, EdU analysis, Transwell experiments, scratch experiments, colony formation assays
Animal Models and Pathological Analysis: Nude mouse in vivo and in vitro cisplatin treatment experiments, animal proliferation and metastasis studies

-
MiR-297 inhibits liver cancer tumor progression by targeting PTBP3
-
Molecular Cancer Review: m6A-regulated tumor glycolysis “collection”
-
Discovery of new biomarkers predicting HCC radiosensitivity! Also related to ferroptosis!
-
5-Nonloxytryptamine mediates MHC-I upregulation in tumors—releasing T cell anti-tumor immunity
-
DRP1-driven mitochondrial plasticity-induced metabolic reprogramming reduces breast cancer brain metastasis
-
Single-cell sequencing reveals multicellular morphology and interactions in atopic dermatitis