*For Medical Professionals Only


The clinical characteristics and imaging manifestations of myocarditis can overlap with other inflammatory or arrhythmic cardiomyopathies. Desmoplakin (DSP) is an important cardiac structural protein. Mutations in the DSP gene are associated with a variant of arrhythmogenic right ventricular cardiomyopathy (ARVC). Interestingly, this unique genetic cardiomyopathy may also present with myocarditis and fibrosis phenotypes, which may resemble other forms of myocarditis (including viral myocarditis), posing challenges for clinical diagnosis.
Recently, the European Heart Journal – Case Reports reported two cases of desmoplakin cardiomyopathy that were initially misdiagnosed as COVID-19 related myocarditis. Can you accurately diagnose and manage these cases? Let’s take a look together.
Case Introduction
Case 1
A 21-year-old female patient presented with intermittent chest pain, described as pressure-like, radiating to both upper limbs, and sought treatment at an external hospital.
Her past medical history was unremarkable. Notably, there was a family history; her 16-year-old brother was suspected to have “viral myocarditis” and related VT. The cardiovascular examination revealed no abnormalities.
Initial troponin I was significantly elevated (23.25 ng/mL; normal value <0.05 ng/mL).
Transthoracic echocardiography showed normal left ventricular systolic function, with an LVEF of 55%, and a small amount of pericardial effusion.
Despite the absence of common COVID-19 related symptoms, the patient’s PCR test for COVID-19 antigen was positive.
The patient discharged herself and presented to our hospital the next day.
High-sensitivity troponin-T was significantly elevated (693 ng/L; normal value <13 ng/L),
and the electrocardiogram showed nonspecific ST segment and T wave abnormalities, including anterior wall T wave inversion (Figure 1).
Repeat COVID-19 antigen testing was positive, and CRP and high-sensitivity CRP were within normal ranges.
Considering the possibility of acute pericarditis related to COVID-19, further cardiac magnetic resonance imaging (CMR) showed mild left ventricular dilation [left ventricular end-diastolic volume (LV EDV) index=85 mL/m2], LVEF of 51%, and mild hypokinesis of the mid and inferior lateral walls. Right ventricular (RV) size and systolic function were normal.
Subepicardial late gadolinium enhancement (LGE) was observed in the left ventricular inferior wall, inferior lateral wall, anterior lateral wall, and anterior wall, with mild pericardial involvement; LGE indicated pericarditis (Figure 2). T1 relaxation time was prolonged (1177 ms, normal <1100 ms), while T2 time was within normal range (52 ms, normal < 60 ms).
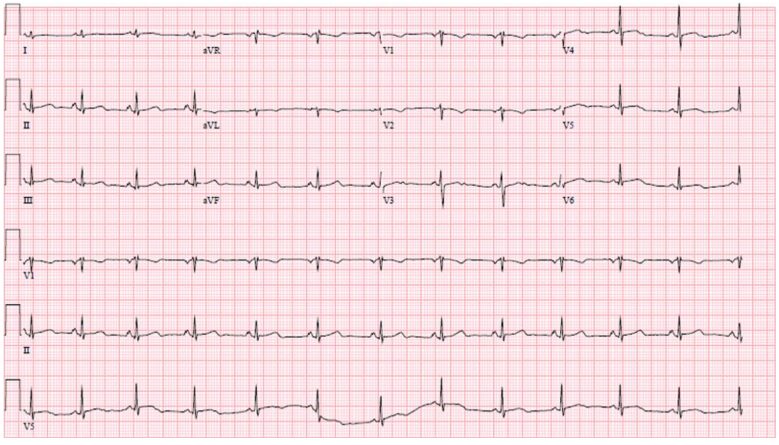 Figure 1 Electrocardiogram
Figure 1 Electrocardiogram
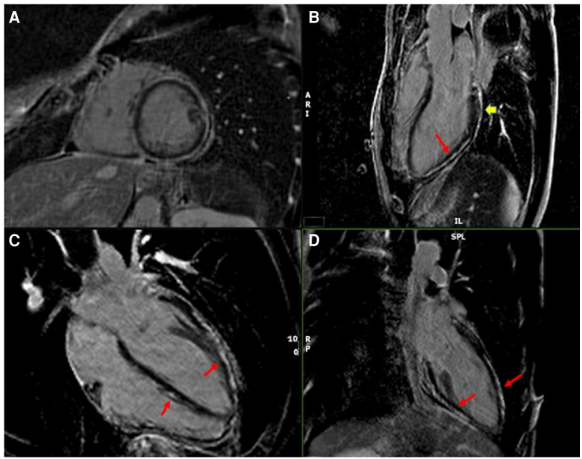 Figure 2 Late gadolinium-enhanced cardiac magnetic resonance imaging. (A) Short axis, (B) three-chamber view, (C) four-chamber view, (D) two-chamber view. Long arrows indicate myocardium, short arrows indicate epicardial gadolinium enhancement
Figure 2 Late gadolinium-enhanced cardiac magnetic resonance imaging. (A) Short axis, (B) three-chamber view, (C) four-chamber view, (D) two-chamber view. Long arrows indicate myocardium, short arrows indicate epicardial gadolinium enhancement
The patient received treatment with beta-blockers, angiotensin-converting enzyme inhibitors, and colchicine, and during follow-up, troponin levels normalized. A 7-day Holter monitor showed 5 episodes of non-sustained ventricular tachycardia.
Considering the family history of arrhythmogenic myocarditis in the patient’s brother, genetic testing was performed, revealing a “likely pathogenic” heterozygous mutation in the DSP gene (c.1267-2A> G variant) (based on the 2015 American College of Medical Genetics and Genomics guidelines, “likely pathogenic” indicates >90% probability that a variant is pathogenic) and a variant of uncertain significance in the Lamin protein gene (c.1210T> A variant).
The patient declined the recommendation for subcutaneous ICD implantation.
One year later, LVEF remained stable, and she continued on medical therapy.
The patient’s brother declined the recommended genetic testing.
Case 2
A 34-year-old male patient, previously healthy, presented as a resident physician with sustained ventricular tachycardia and syncope.
Cardiac examination: regular rhythm, no valve murmurs, pericardial friction rub, or gallop rhythm; the point of maximal impulse was displaced laterally. Jugular venous pressure was not elevated, and there was mild edema in both lower limbs.
The electrocardiogram showed nonspecific ST segment and T wave abnormalities, including T wave inversion in the inferior and anterolateral walls (Figure 3).
High-sensitivity troponin-T was within normal range (11 ng/L; normal <21 ng/L).
The patient started amiodarone to control VT episodes. He denied any recent symptoms related to COVID-19, and both COVID-19 antigen and antibody tests were negative. CRP levels were also within normal ranges.
Transthoracic echocardiography showed severe left ventricular dilation and overall decreased systolic function (left ventricular end-diastolic diameter 6.6 cm, LVEF 20%).
Coronary CTA showed no evidence of coronary artery atherosclerosis.
Cardiac magnetic resonance imaging again demonstrated significant left ventricular dilation and severe overall systolic dysfunction (LV EDV index=143 mL/m2), with normal right ventricular size and mildly reduced systolic function (right ventricular EDV index=66 mL/m2, RVEF=41%).
Mid-myocardial LGE involved the anterior septum and inferior septum, with epicardial LGE centered on the anterior lateral and inferior lateral walls supporting a myocarditis-pericarditis pattern (Figure 4). Enhanced T1 relaxation time was prolonged (1155 ms, normal <1100 ms), and enhanced T2 relaxation time was within normal range (45 ms, normal <60 ms).
Considering the history of sustained ventricular tachycardia, the patient underwent subcutaneous ICD implantation and endomyocardial biopsy, which revealed lymphocytic myocarditis and fat infiltration related to ARVC (Figure 5).
Despite receiving medical therapy, due to persistent VT, steroid therapy was initiated.
Further evaluation with 18F-fluorodeoxyglucose PET (FDG-PET) showed no active inflammation.
Genetic testing revealed a “pathogenic” heterozygous DSP mutation (c.2185dup variant).
The patient experienced intermittent episodes of ventricular fibrillation and was defibrillated once by his ICD. Therefore, he underwent epicardial VT ablation targeted at the apical epicardial isthmus.
The patient continued on beta-blockers, angiotensin receptor blockers, mineralocorticoid receptor antagonists, sodium-glucose co-transporter inhibitors, and amiodarone to suppress VT treatment.
The patient was able to return to work and had no further VT episodes.
One year later, follow-up echocardiography showed LVEF still significantly reduced but stable.
Genetic testing was recommended for the patient’s first-degree relatives.
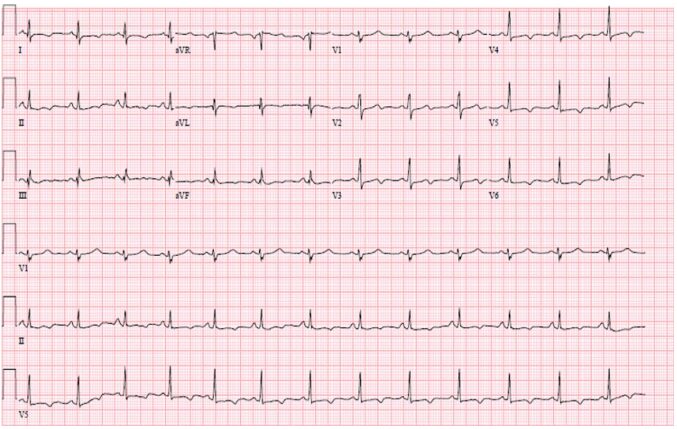
Figure 3 T wave inversion in leads I, aVL, II, aVF, and V3-V6.
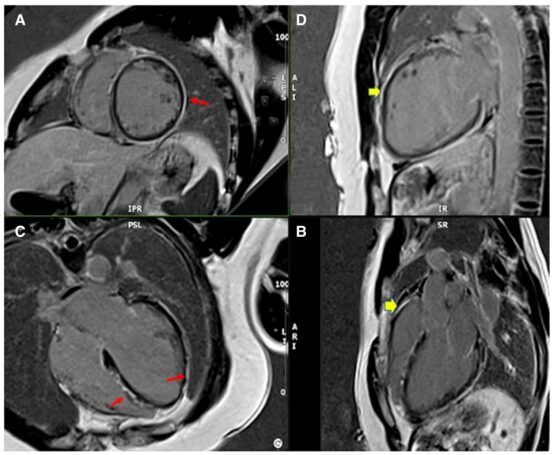
Figure4 Late gadolinium-enhanced cardiac MRI images of patient 2.(A)Short axis, (B)Two-chamber view, (C)Four-chamber view, (D)Three-chamber view. Long arrows indicate myocardium, short arrows indicate epicardial gadolinium enhancement.
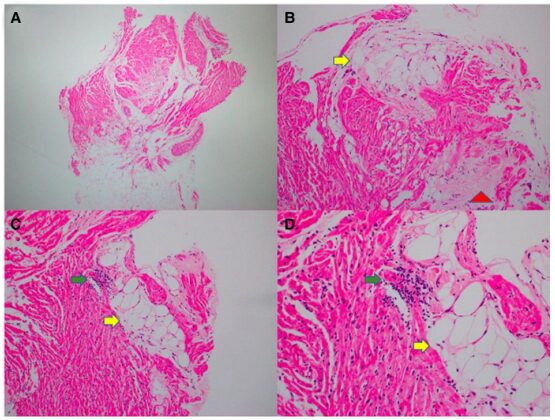
Figure5 Endomyocardial biopsy of patient 2. Low-power (A) and medium-power (B-D) images of hematoxylin and eosin staining show mild myocardial cell hypertrophy, fibrotic disarray, and scattered lymphocytic infiltration (green arrows) adjacent to fat deposition (yellow arrows) and interstitial collagen fibrosis patches (red arrows).
Discussion
Desmoplakin is a cardiac structural protein that is crucial for normal force transmission in the myocardium. DSP gene mutations are associated with arrhythmogenic cardiomyopathy, which is classified as LDAC, a variant of ARVC primarily affecting the left ventricle. Patients carrying these mutated genes typically exhibit left ventricular systolic dysfunction and frequent clinical manifestations of premature ventricular contractions and ventricular tachycardia.
Furthermore, an inflammatory component leading to fibrosis may contribute to the development and progression of DSP cardiomyopathy. This inflammatory component may resemble and confound other forms of myocarditis, including viral myocarditis, which poses challenges for the diagnosis and treatment of these patients. Specifically, DSP cardiomyopathy needs to be considered in the differential diagnosis of myocarditis, as the pattern of LGE on CMR is nearly indistinguishable between the two. Additionally, during the current COVID-19 pandemic, this myocarditis-like presentation may have particular clinical significance, where COVID-19 related myocardial injury, including acute myocarditis, has been a concern.
DSP gene mutations were first identified in an autosomal recessive form, but these mutations have a high clinical penetrance in carriers, who may exhibit prominent phenotypic features. A multicenter cohort study by Smith et al. involving 107 DSP mutation patients (including probands and their genotype-positive family members) found that 69% of patients were female, and 55% had characteristic curly hair and/or thick skin on palms or soles (palmoplantar keratoderma).
Although often classified as ARVC, left ventricular involvement is more common in DSP cardiomyopathy; therefore, Sen-Chowdhry et al. proposed the term LDAC. In 2008, Smith et al. reported that among their 107 subjects, 51% had predominant left ventricular involvement, 14% had predominant right ventricular involvement, 4% had isolated RV cardiomyopathy, while 36% had normal function in both left and right ventricles. More probands had left ventricular dysfunction compared to genotype-positive family members.
Cardiac magnetic resonance imaging plays an important role in the diagnosis of DSP cardiomyopathy. Similar to the cases presented above, Smith et al.’s study group showed that 74% of left dominant cardiomyopathy patients exhibited left ventricular LGE among all subjects who underwent CMR. LGE is commonly seen in the subepicardial inferior segment, with some cases extending to the myocardium of the interventricular septum. In some cases, subepicardial LGE may present with a circular distribution and fat within or adjacent to the fibrotic areas of the epicardium.
Histopathological analysis of autopsy samples from cases of sudden cardiac death related to LDAC has also reported this distribution of intramyocardial fat. Despite being a genetic cardiomyopathy, patients with DSP cardiomyopathy may exhibit unique clinical features of acute myocardial injury and inflammation, including chest pain and elevated troponin, as seen in case 1.
Smith et al. reported that 15% of patients with DSP mutations exhibited evidence of acute myocardial injury, most of whom (9/10) presented with left ventricular LGE on CMR. Acute myocardial injury can occur even with normal systolic function; they suggested that myocardial injury and fibrosis may precede ventricular systolic dysfunction. The proportion of non-probands presenting with chest pain, elevated troponin, and left ventricular LGE was similar to that of probands (13% vs. 18%, respectively).
In contrast, in their cohort, 4 patients with elevated troponin underwent cardiac FDG-PET scanning, showing active myocardial inflammation. These features differ from ARVC, where the 2010 revised ARVC Working Group criteria proved insensitive for clinical diagnosis in DSP mutation patients, even among a few patients with predominant RV involvement. The inflammatory process as a precursor to myocardial fibrosis and heart failure makes inflammatory signaling pathways attractive new therapeutic targets for DSP cardiomyopathy patients.
The cases presented above demonstrate that DSP cardiomyopathy is associated with an increased risk of ventricular arrhythmias and sudden cardiac death. LVEF <55% and frequent premature ventricular contractions are strong predictors of these events. Unlike other forms of dilated cardiomyopathy, an LVEF threshold <35% is considered an insensitive indicator of future arrhythmic events in DSP cardiomyopathy.
Desmoplakin gene mutations have a high clinical penetrance; therefore, family members of patients with DSP cardiomyopathy are at significant risk for adverse events, highlighting the need for family screening using genetic testing, CMR (to assess left ventricular LGE, regardless of left ventricular echocardiographic function), and heart rate monitoring to evaluate PVC burden.
References
[1] A case series of desmoplakin cardiomyopathy: a mimic of viral myocarditis. Eur Heart J Case Rep. 2022;6(8):ytac341. doi: 10.1093/ehjcr/ytac341
[2] Research progress on arrhythmogenic cardiomyopathy with left ventricular involvement. Chinese Circulation Journal, 2016, 31(09):931-934.
2022 ACC Expert Consensus | How to Perform Emergency Evaluation and Treatment of Acute Chest Pain?
Progress | JAMA Sub-journal: Qualitative Analysis of the Doctor-Patient Communication Process in Atrial Fibrillation Anticoagulation Decision-Making
Progress | NEJM: Treatment of Resistant Hypertension, New Selective Aldosterone Synthase Inhibitor Baxdrostat or Effective
Progress | NEJM: Mechanical Ventilation Treatment in Severe Adult Patients, What is the More Appropriate Oxygen Saturation Target?
Progress | NEJM: Limited Benefit of Pemafibrate in Reducing Triglycerides in Patients on Statin Therapy
Progress | Effects of Canagliflozin on Cardiovascular Outcomes at Different Renal Function and Albuminuria Levels
Progress | JACC: Reduction in Cardiovascular Risk Based on Time Within Treatment Range After Catheter Renal Denervation
Progress | Atrial Fibrillation Ablation and Esophageal Injury Prognosis: High-Power Short Duration vs Low-Power Long Duration Posterior Wall Ablation
Progress | Can siRNA Inclisiran Reduce Cardiovascular Events? A Patient-Level Data Analysis of a Phase III Trial
Progress | Catheter Ablation Improves Cardiovascular Prognosis in Patients with Atrial Fibrillation and Heart Failure: A Meta-Analysis of Randomized Controlled Trials
Case | How to Prevent Cardiovascular Disease in Patients with Elevated Lp(a)?

