Click the above “CAIVD” to follow us
In Vitro Diagnosis, or IVD, refers to obtaining clinical diagnostic information by testing human samples (such as blood, body fluids, tissues, etc.) outside the human body, thereby assessing diseases or bodily functions. The instruments, reagents, and consumables required during the testing process constitute the IVD system, while companies engaged in the research, production, and marketing of these instruments, reagents, and consumables form the IVD industry. With the development of society, higher demands are placed on the IVD industry, requiring both rapid testing and precise measurement results to secure precious time for clinical diagnosis and treatment.
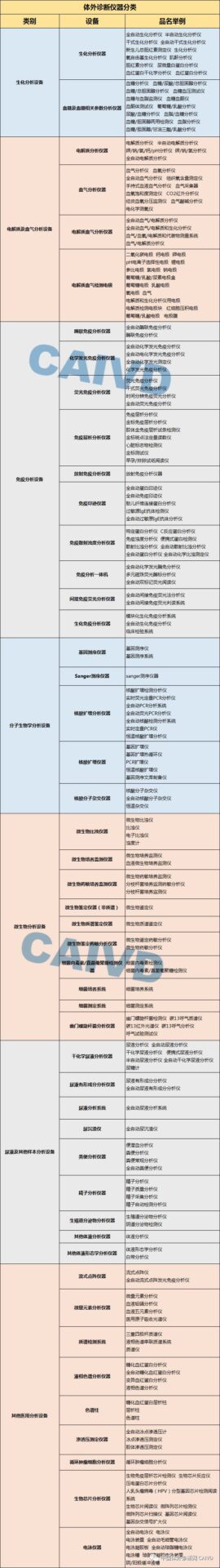
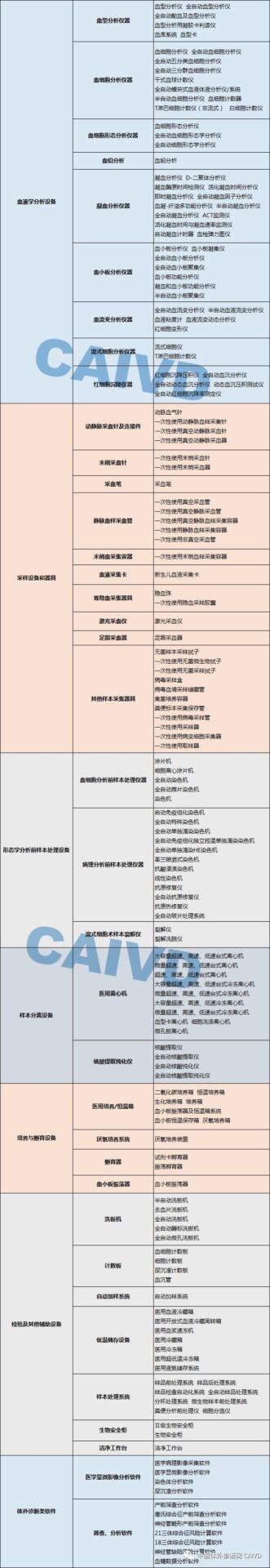
In Vitro diagnostic instruments are mainly divided into: biochemical analysis equipment, electrolyte and blood gas analysis equipment, immunoassay equipment, molecular biology analysis equipment, microbiological analysis equipment, urine and other sample analysis equipment, other medical analysis equipment, hematology analysis equipment, sampling devices and tools, sample processing equipment for morphological analysis, sample separation equipment, cultivation and incubation equipment, inspection and other auxiliary equipment, and IVD-related software.
The editor has compiled a list of commonly used laboratory equipment for reference:
Related Reading
Classification of In Vitro Diagnostic Reagents (Collection)
Contributed by: Ice Tea
Editor: Yi Shui
Proofread by, Chief Editor: Sun Xuguang

Recommended Reading
▶Interview with a Leader: Dr. Jiang Zhiming, Global Vice President and General Manager of Beckman Coulter China
▶Interview with a Leader: Dr. Chen Lili, Chairman and General Manager of Wuhan Mingde Biotechnology Co., Ltd.
▶Song Haibo: Current Status and Considerations of Key Raw Materials for In Vitro Diagnosis in China
▶Summary of Registration Information for Class I Medical Devices of In Vitro Diagnostic Products in the First Half of 2019
▶Summary of Diagnosis and Treatment Norms Related to In Vitro Diagnosis in 2018
▶Another In Vitro Diagnosis Related Association Established (with a list of 44 National In Vitro Diagnosis Related Associations)
