Original Source
Park JW, Sahm F, Steffl B, Arrillaga-Romany I, Cahill D, Monje M, Herold-Mende C, Wick W, Turcan Ş. TERT and DNMT1 expression predict sensitivity to decitabine in gliomas. Neuro Oncol. 2021 Jan 30;23(1):76-87.
Research Background
Adult diffuse gliomas are mainly classified into three types: isocitrate dehydrogenase (IDH) mutant gliomas with chromosome arm 1p/19q codeletion (oligodendrogliomas, IDH-mutant-codel), IDH mutant gliomas with intact 1p/19q (astrocytomas, IDH-mutant-noncodel), and IDH wild-type gliomas. The DNA methyltransferase inhibitor (DNMTi) decitabine (DAC) effectively treats gliomas by incorporating into DNA, making it a more effective demethylating agent that inhibits DNA methyltransferase, leading to DNA demethylation and cellular differentiation or apoptosis.
Telomeres are repetitive DNA sequences at the ends of eukaryotic linear chromosomes that protect the chromosome ends from fusion and degradation. Due to the end replication problem, telomeres gradually shorten with cell division. Telomerase is responsible for repairing and extending telomeres, including telomerase reverse transcriptase (TERT) and its RNA component (TERC). Previous studies have shown that in IDH-mutant-codel gliomas with TERT promoter (TERTp) mutations, DAC can downregulate TERT expression, but the mechanism of TERT downregulation remains unclear. The authors elucidated the mechanism by which DAC inhibits TERT and confirmed that the efficacy of DAC is associated with TERT and DNMT1 levels in both IDH mutant and IDH wild-type glioblastomas.
Research Results
Result 1: DAC treatment downregulates DNMT1 levels, cell proliferation, and anchorage-independent growth in IDH-mutant-codel gliomas.
The authors examined four glioma cell lines (TS603, NCH612, SU-AO3, and NCH1681) and found that they all contained endogenous IDH1 R132H mutations (Fig. 1A). After DAC treatment of TS603 cells, DNMT1 protein expression, cell proliferation, and anchorage-independent growth activity decreased (Fig. 1B-D). To study the role of DAC-mediated growth inhibition in other types of gliomas, the authors examined NCH612 (1p/19q partial codeletion) and SU-AO3 (1p/19q complete codeletion). The results showed that DAC downregulated DNMT1 protein expression (Fig. 1E), and after 7 days of DAC treatment, the colony formation ability of NCH612 and SU-AO3 significantly decreased (Fig. 1D). The authors then studied the sensitivity of IDH1 mutant gliomas to DAC by measuring cell proliferation rates. The results indicated that compared to TS603 cells, DAC had a more significant inhibitory effect on the colony formation and proliferation of NCH612 and SU-AO3 cells (Fig. 1G-I). However, DAC had no effect on the proliferation of NCH1681 cells (IDH-mutant-noncodel, grade III astrocytoma) (Fig. 1F-J), suggesting that DAC’s inhibitory effect on cell proliferation is more pronounced in IDH-mutant-codel gliomas.
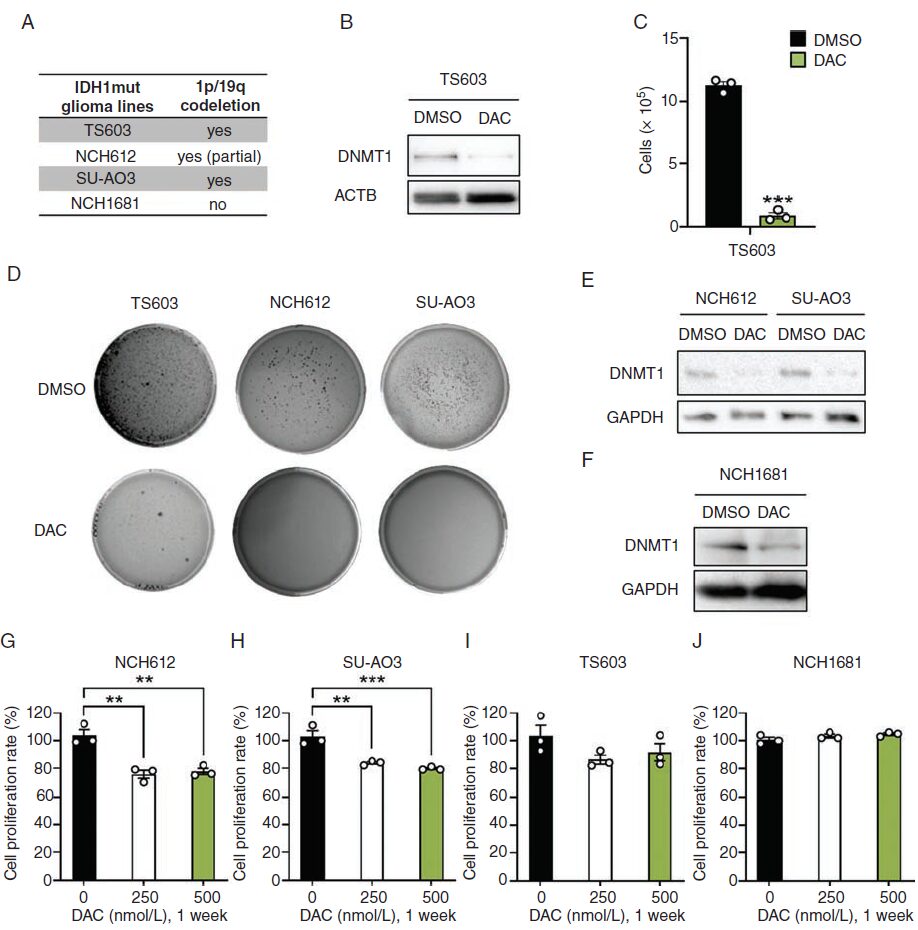
Fig. 1
Result 2: DAC treatment upregulates DNA replication, lysosomal, and cell cycle pathways in IDH-mutant-codel gliomas.
To evaluate the impact of DAC on gene expression in IDH-mutant-codel glioma cells (SU-AO3, NCH612, and TS603), the authors treated the cells with a low dose (500nM) of DAC for 7 days and identified differentially expressed genes through RNA sequencing. KFGG enrichment analysis revealed that DNA replication, lysosomal pathways, and cell cycle pathways were enriched in the three DAC-sensitive IDH-mutant-codel glioma cells, while not enriched in IDH-mutant-noncodel gliomas. DAC upregulated the p53 signaling pathway in IDH1 mutant glioma cells (Fig. 2A).
Result 3: DAC-induced upregulation of p21 expression is negatively correlated with TERT expression.
To study the gene regulatory mechanisms of DAC treatment on tumors, the authors investigated overlapping genes in SU-AO3, NCH612, and TS603 (Fig. 2B). Based on KEGG analysis, they found cell cycle-related cyclin-dependent kinase inhibitor 1A (CDKN1A; p21), CDT1, GADD45A, MCM5, PLK3, and TERT among these genes (Fig. 2C). Immunoblotting and qRT-PCR confirmed that DAC treatment increased p21 expression and decreased TERT expression in IDH-mutant-codel glioma cells (Fig. 2D and E). The expression of TERT and CDKN1A (p21) was negatively correlated (Fig. 2F), indicating a correlation between DAC-induced activation of p21 and inhibition of TERT.
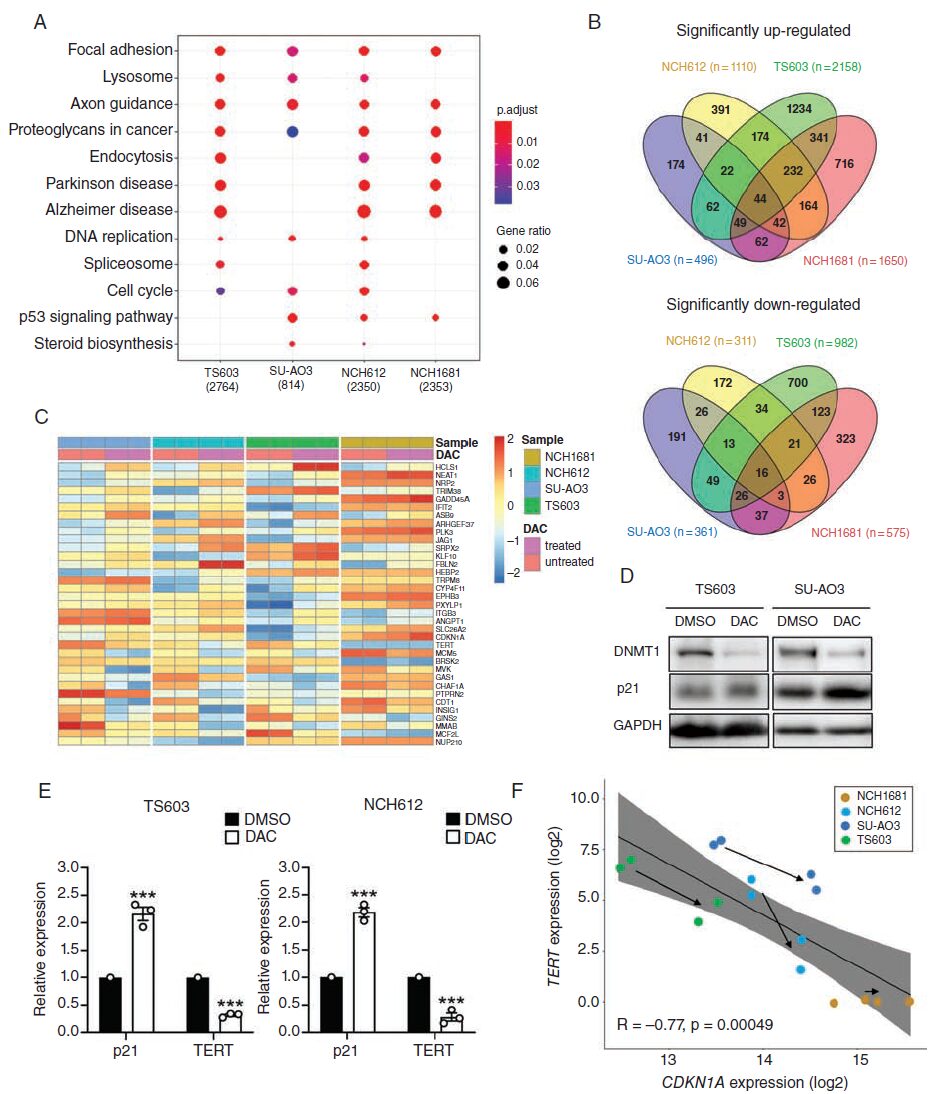
Fig. 2
Result 4: DAC inhibition of TERT depends on the p53/p21 pathway.
The authors further examined the levels of p21 and TERT after DAC withdrawal, observing an increase in TERT expression on day 25 after DAC withdrawal in SU-AO3 and TS603 cells, with a significant decrease in p21 expression compared to the initial levels after DAC treatment (Fig. 3A), indicating that the changes in p21 and TERT levels induced by DAC are reversible. Moreover, after DAC withdrawal, the cells remained sensitive to subsequent DAC treatment (Fig. 3B).
To clarify whether p53 is involved in the regulation of p21, the authors pretreated SU-AO3 cells with PFTα (a p53 inhibitor) for 24 hours before DAC treatment, finding that p21 expression was suppressed (Fig. 3C), indicating that DAC-induced p21 expression is regulated by p53, and that pretreatment with PFTα can eliminate the proliferation defects induced by DAC (Fig. 3D). To explore the role of p53 in DAC inhibition of TERT, the authors examined the levels of p21 and TERT after doxorubicin treatment. Similar results were observed in doxorubicin-treated cells (Fig. 3E). To determine whether DAC inhibition of TERT requires p21 involvement, the authors constructed TS603 cells with p21 shRNA and demonstrated that p21 knockout led to a significant upregulation of TERT (Fig. 3F).
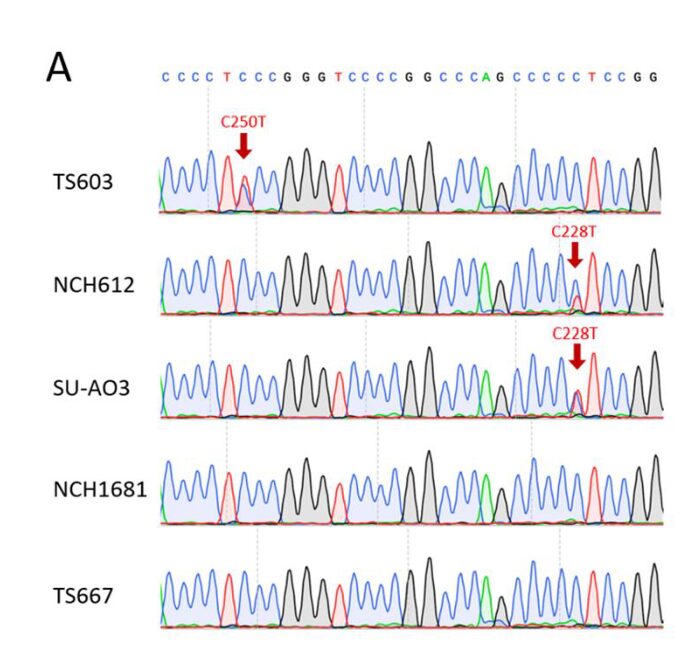
Fig. 3
Result 5: DAC-mediated proliferation defects depend on TERT expression.
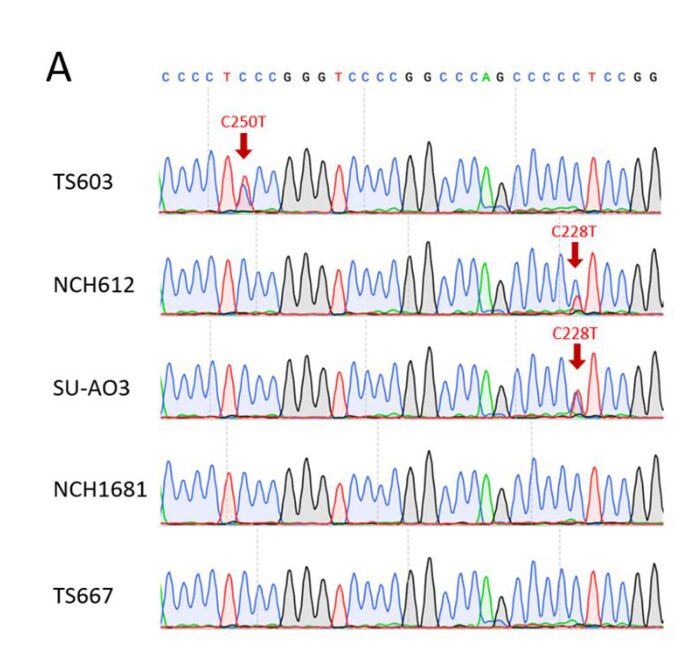
The authors evaluated the impact of IDH1 mutation on DAC sensitivity in TERT IHA cell lines with high expression of IDH1 R132H. In addition to the IDH mutant IHAs, the parental IHAs also exhibited significant DAC response (Fig. 4A). TERTp mutations frequently occur in oligodendrogliomas (ODG) and glioblastomas (GBM). Sanger sequencing revealed two hotspot mutations in TERTp (C228T or C250T) in IDH-mutant-codel glioma cells (Supplementary Fig. 3A). To determine whether DAC-mediated proliferation defects depend on TERT expression, the authors constructed TS603 and TS667 cell lines to overexpress TERT using a TERT-HA expressing retrovirus (Fig. 4B). The results showed that TERT overexpression significantly enhanced the sensitivity of both glioma cell lines to DAC (Fig. 4C and D). Additionally, the use of the small molecule TERT inhibitor BIBR1532 reduced the sensitivity of TS603 and TS667 to DAC, indicating that TERT is necessary for DAC-induced proliferation defects (Fig. 4C and D). Although either BIBR1532 or DAC alone significantly inhibited the proliferation of NCH612 and SU-AO3 cells, the combination of BIBR1532 and DAC did not enhance or synergize, indicating that TERT inhibitors interfere with DAC efficacy, and vice versa (Fig. 4E and F). To investigate whether changes in telomerase activity affect glioma cell sensitivity to DAC, the authors treated the cells with the telomerase inhibitor 6-thio-dG and found that the proliferation defects were not improved (Fig. 4G and H). In summary, TERT likely influences glioma cell sensitivity to DAC through a telomerase-independent mechanism.
Supplementary Fig. 3
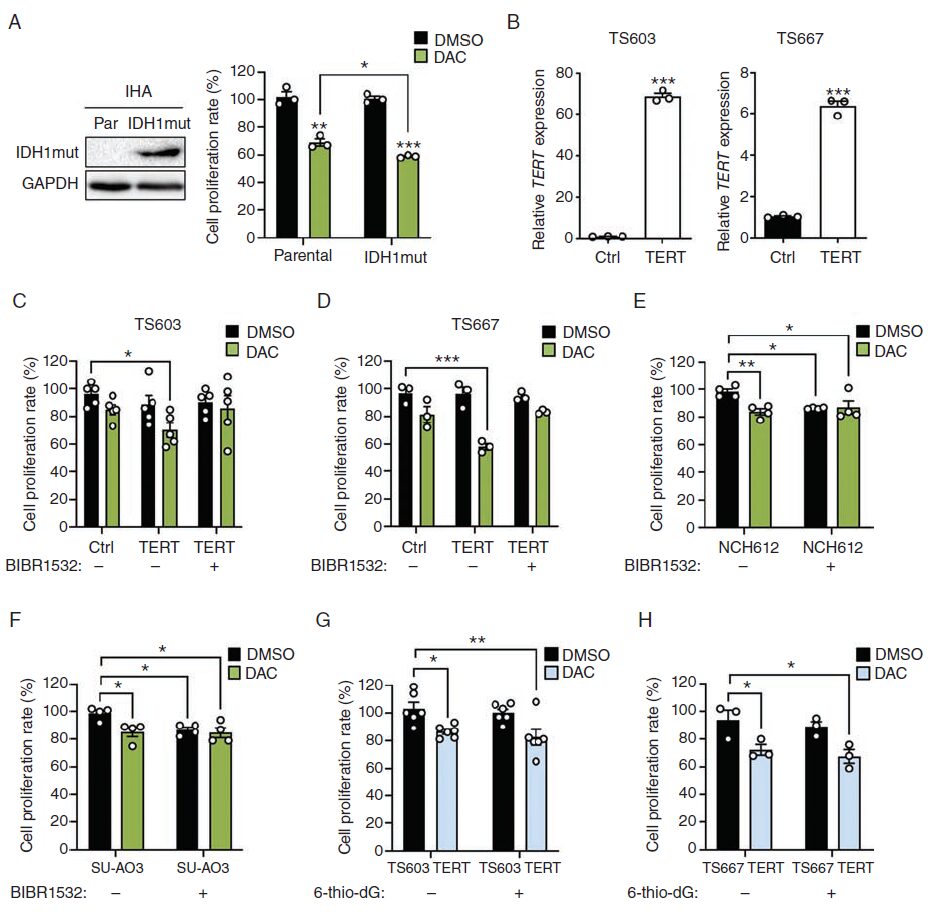
Fig. 4
Result 6: Expression of TERT and DNMT1 in IDH wild-type GBM correlates with DAC response.
Given the high expression of TERTp mutations in oligodendrogliomas and 80% of IDH wild-type gliomas, the authors investigated whether TERT expression could predict the sensitivity of IDH wild-type gliomas to DAC. By querying the TCGA database, they found high expression of TERT in the classical GBM subtype with EGFR amplification and mutations (Supplementary Fig. 4A). The authors evaluated the efficacy of DAC in six GBM cell lines with or without EGFR overexpression and observed different DAC responses in GBMs similar to IDH1 mutant gliomas (Fig. 5A). DAC had the strongest growth inhibitory effect on L0627 cells with high TERT expression, and hotspot mutations in TERTp were present in all GBM cell lines. To further determine the molecular determinants of glioma response to DAC, the authors studied overlapping genes associated with TERT expression from the TCGA database, which were related to IDH-mutant-codel gliomas and classical glioma subtypes (Supplementary Fig. 4B). Additionally, DNMT1 and DNMT3B were highly expressed in the classical subgroup of IDH wild-type GBM (Supplementary Fig. 4C and D). Given that DAC is an inhibitor of DNMTs, the authors measured the levels of endogenous DNMT1 and DNMT3B in GBM. The results indicated that DNMT1 expression was associated with DAC response, while DNMT3B expression was not (Fig. 5A-C). These data suggest that the expression of TERT or DNMT1 may help predict DAC efficacy.
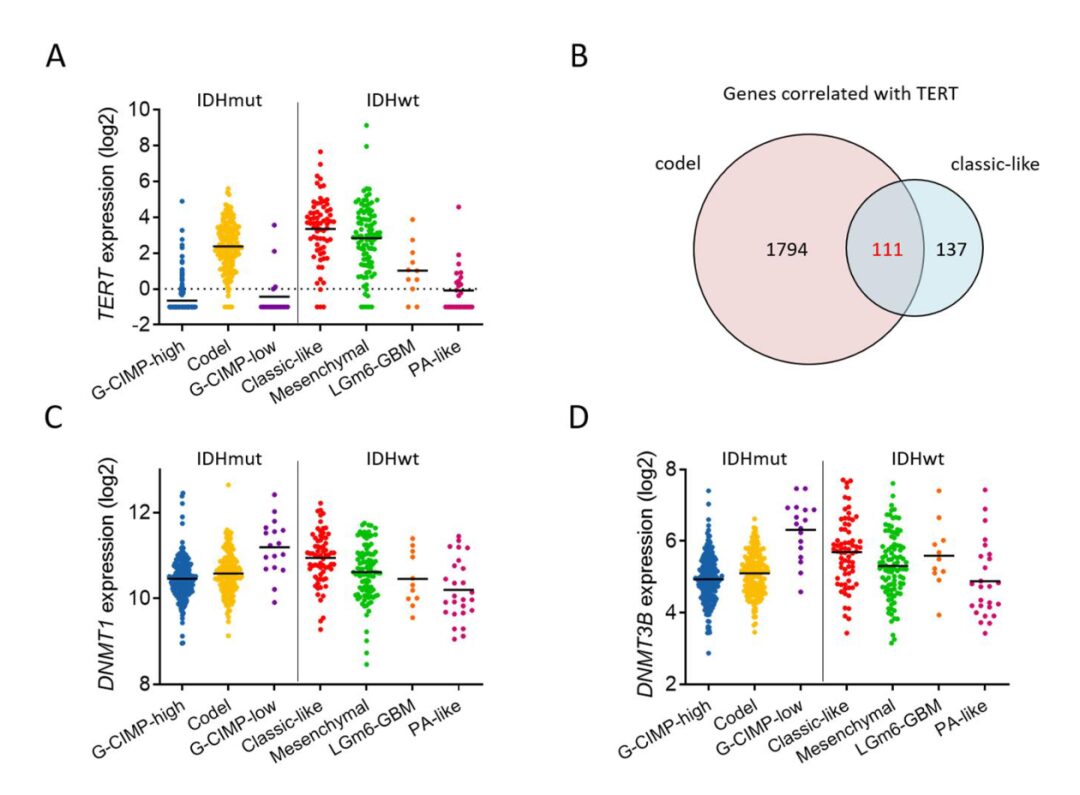
Supplementary Fig. 4
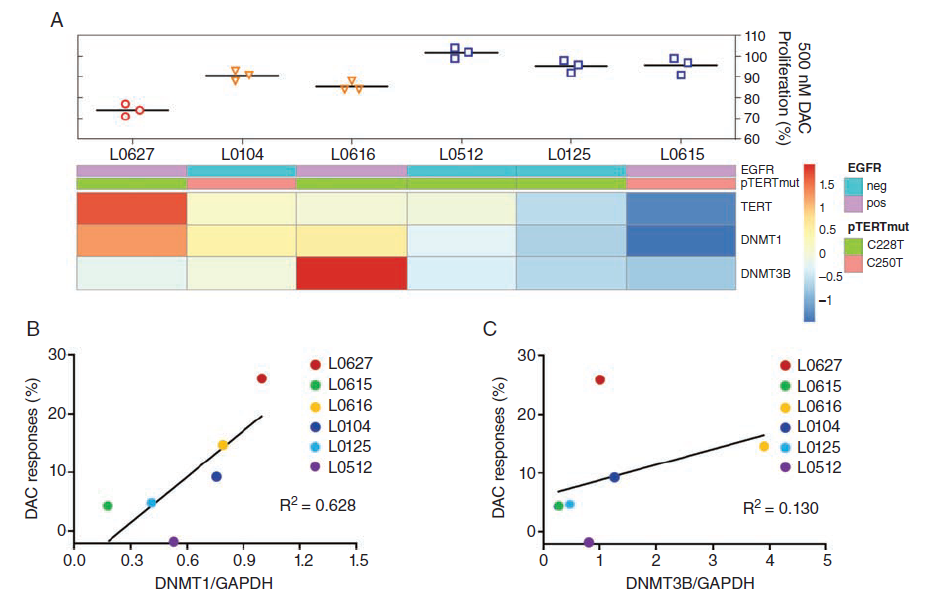
Fig. 5
Result 7: DNMT1 plays an important role in DAC treatment in gliomas with high TERT expression.
Previous studies have shown that DNMT1 protein levels are closely related to DAC responses in embryonic stem cells and breast cancer organoids. Since DNMT3A and DNMT3B exhibited low protein and gene expression levels in four IDH1 mutant strains, and DNMT3B expression in GBM had little correlation with DAC treatment, the authors focused on DNMT1 protein levels to determine whether its expression could serve as a potential biomarker for DAC efficacy in gliomas. The authors demonstrated that in DAC-sensitive cells (SU-AO3, NCH612, L0627, and L0616), DNMT1 levels were higher, indicating that DNMT1 protein levels are closely related to DAC responses in gliomas (Fig. 6A and B).
Given that TERT can stimulate the expression of DNMT3B in hepatocellular carcinoma, the authors verified whether TERT overexpression would enhance DNMT1 expression levels. The results indicated that DNMT1 protein expression increased in TERT-overexpressing TS603 cells (Fig. 6C). The authors then hypothesized that TERT-induced DNMT1 may lead to increased sensitivity to DAC. To verify whether DNMT1 knockout inhibited TERT-mediated DAC response, the authors knocked down DNMT1 expression using shRNA and found that in DNMT1 knockout cells, cell proliferation activity significantly decreased (Fig. 6D-F), and cells with low DNMT1 levels were insensitive to DAC treatment, while DNMT1 knockout protected against DAC-mediated proliferation defects (Fig. 6D-F).
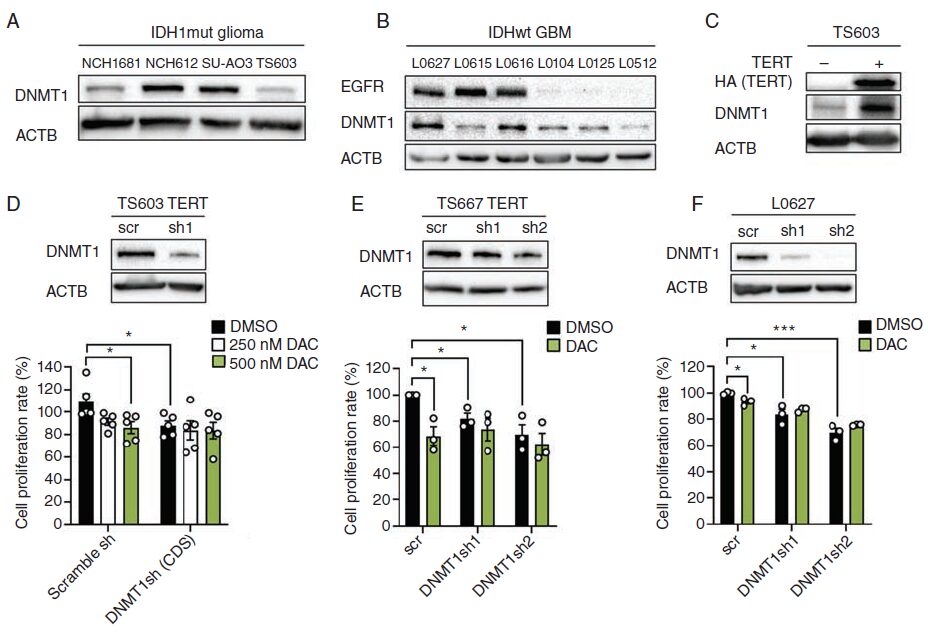
Fig. 6
Research Conclusion
Gliomas with chromosome arm 1p/19q codeletion and IDH1 mutations are sensitive to DAC treatment. DAC inhibits TERT activity by upregulating the expression of cyclin-dependent kinase inhibitor CDKN1A (p21). p21 gene knockout leads to upregulation of TERT expression, and TERT overexpression enhances DNMT1 expression levels and DAC sensitivity through a telomerase-independent mechanism. Furthermore, shDNMT1 can reduce DAC efficacy in TERT-overexpressing glioma cells, indicating that DNMT1 is necessary for DAC action in TERT-overexpressing gliomas, and the expression levels of TERT and DNMT1 are potential factors determining DAC treatment efficacy.
Proofread by: Liu Mingdi









