Skip to content
Electrocatalysis Discussion Group-1: 529627044
Special Note:This article is originally written by MeLab Technology Center, aiming to share relevant scientific research knowledge. Due to limited knowledge, there may be omissions and errors, please read critically, and kindly ask for corrections from knowledgeable readers.
Atomically dispersed single-atom catalysts (SAC) have gained widespread research attention in recent years due to their high atomic utilization, unique electronic structures, and well-defined active sites. However, low metal loading, weak interactions between isolated metal atoms, and a lack of adsorption sites limit their application in many complex reactions. Dual-atom catalysts (DAC) with high activity and atomic utilization are expected to become the new favorites in sustainable energy conversion and storage technologies.
However, the design of dual-atom catalysts still faces the following issues:
1. Current methods for preparingDAC face the issue of structural inhomogeneity.
DACS can be prepared through various synthesis routes that depend on confinement or molecular anchoring effects. However, the limited “precision engineering” capabilities in current methods for preparing DAC lead to structural inhomogeneity, making it difficult to accurately distinguish target dual-atom pairs from dense atoms, resulting in undesirable side reactions.
2. Difficulty in precisely controlling the individual coordination of two different metal atoms.
To maximize the potential ofDAC in multi-step catalytic reactions, it is necessary to customize different anionic ligands for the two metals to constructJanus bimetallic sites. However, due to the lack of effective synthetic methods and the difficulty in distinguishing and identifying the exact amorphous structures of complex active sites, Janus-typeDAC with clearly defined multiple ligands are rarely reported.
In light of this, Yan Wensheng, Tan Hao from USTC, and Wang Dingsheng from Tsinghua University developed a strategy for synthesizing carbon-based catalysts with dual-atomFe-Co sites, whereFe andCo atoms are coordinated withN andO atoms respectively, and connected through bridgingN andO atoms (FeCo-N3O3@C).TheJanus FeCo-N3O3@C tetranuclear dimer is a stable and efficient bifunctional catalyst, which can be used for electrocatalytic redox reactions (half-wave potential E1/2=0.936 V) and oxygen evolution reactions (potentialE=1.528 V at 10 mA cm−2). When assembled in aZn-air battery, it demonstrates superior performance compared to the benchmarkPt/C+RuO2 air cathode. A series of in situ and ex situ characterizations combined with theoretical calculations indicate that the catalyst’s bifunctionality arises from the strong coupling betweenFe–N3 andCo–O3 parts, thereby altering thed orbital energy levels of the metal atoms, optimizing the adsorption-desorption of oxygen-containing intermediates, and improving the reaction kinetics of redox and evolution reactions. Understanding the fundamental mechanisms of this dual-atom catalyst at the atomic and electronic levels will help in the rational design of efficient multifunctional catalysts with customized activity for specific reactions.
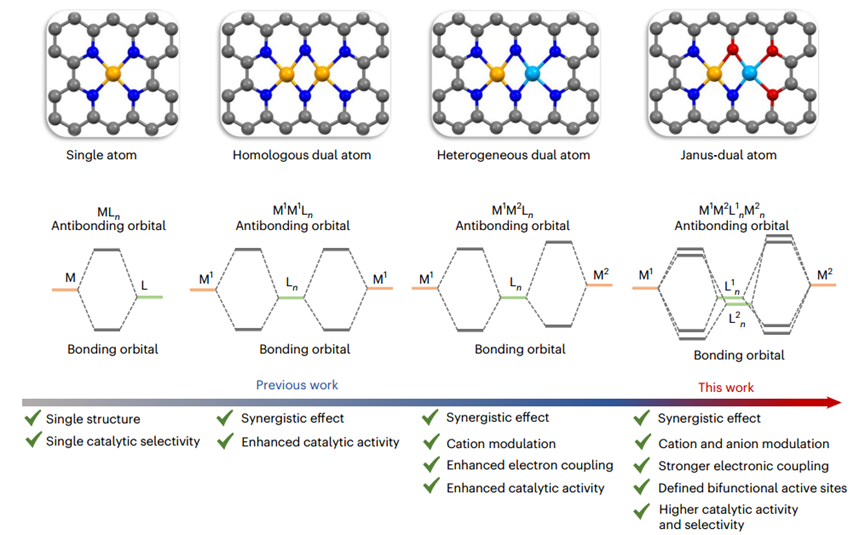 Figure 1 Research on carbon-based atomically dispersed metal catalysts
1. Synthesized and characterizedFeCo–N3O3@C catalyst
The authors developed aDAC synthesis strategy to synthesizeFeCo–N3O3@C Janus DAC nanosheets, and demonstrated the micro-morphology and local coordination environment of the catalyst through various characterization methods.
2. Tested the electrocatalytic performance ofFeCo–N3O3@C DAC.
The authors evaluated the electrocatalytic performance ofFeCo–N3O3@C DAC in an oxygen-saturated0.1 M KOH electrolyte using a typical three-electrode system, confirming its excellent bifunctional catalytic performance.
3. Analyzed the mechanism of electrocatalytic activity
The authors usedDFT calculations to deeply analyze the catalytic mechanisms ofORR andOER, and experimental characterizations confirmed that the strong synergistic interaction between theFe–N3 and Co–O3 parts can enhance the ORR and OER activity by reshaping the charge distribution of the dual-site active centers.
4. Investigated the performance of ZAB
The authors assembled a rechargeable aqueousZAB withFeCo–N3O3@C loaded on the cathode side in an air atmosphere, demonstrating excellent performance and outstanding stability compared to commercial batteries based onPt/C+RuO2.
1. Developed a feasible strategy to construct Janus bimetallic sites
The authors developed a feasible strategy to construct Janus bimetallic sites in efficient bifunctional catalysts, successfully synthesizing a carbon-based catalyst withFe-Co sites, and determined the structure of theFeCo-N3O3@C tetranuclear dimer catalyst through various characterization methods.
2. Achieved a bifunctional catalyst with excellent performance
The catalyst developed by the authors exhibits excellent bifunctional activity, outperforming reported single-atom counterparts. It was validated in a Zn-air battery with a power density of up to143 mW cm-2, and long-term durability exceeding200 hours. The high catalytic activity of theJanus FeCo–N3O3@C catalyst is attributed to the optimized filling of the 3d orbitals ofCo andFe atoms.
Synthesis and characterization of theFeCo–N3O3@C catalyst
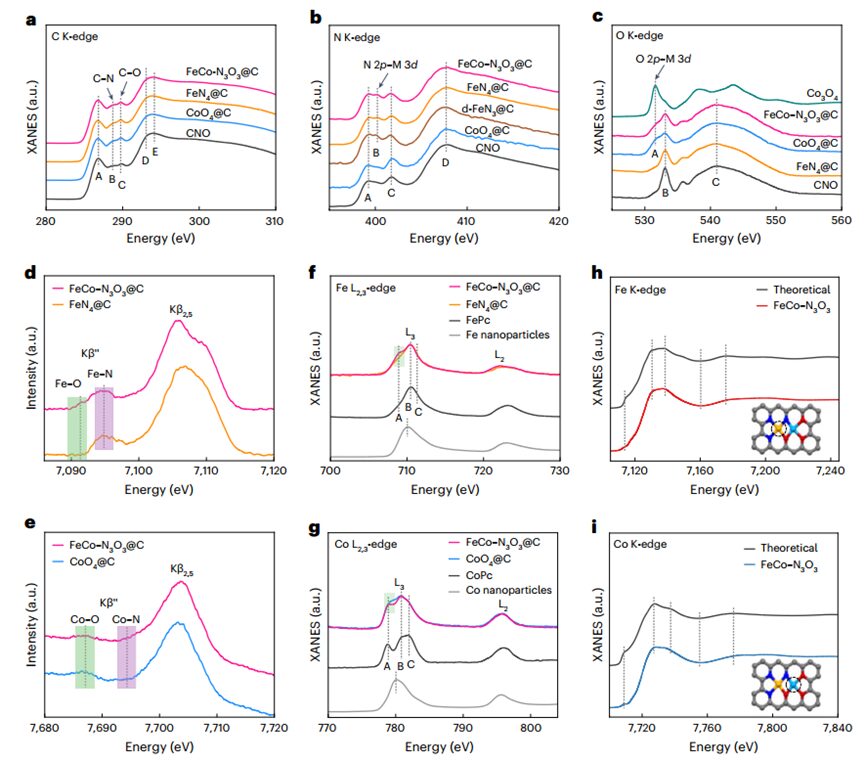
The authors developed aDAC synthesis strategy that allows for the orderly assembly of two different functional components. The article presents the process of synthesizingFeCo–N3O3@C Janus DAC nanosheets through a three-step strategy. The loading ofFe andCo inFeCo–N3O3@C was determined to be1.16 and 0.97 wt% respectively by inductively coupled plasma atomic emission spectroscopy. SEM and TEM showed that all samples inherited a 2D hexagonal sheet morphology from the MOF precursor, with a thickness of about 30-100 nm. HRTEM, SAED, and PXRD did not observe any metal particles or clusters. HAADF-STEM indicated that nearly 100% of theFe atoms exist in single-atom form. After successfully introducing theCo-O3 part, a considerable proportion of bimetallic dimers appeared. The HAADF-STEM image ofFeCo–N3O3@C indicates that high-density heteronuclearFe–Co dual-atom sites were successfully obtained. Furthermore, the local coordination environment ofFe andCo atoms inFeCo–N3O3@C was studied using EXAFS spectroscopy, indicating thatFe species primarily exist in single-atom form inFeN4@C, and form metal-metal bonds after introducingCo–O3. XANES andXES further distinguished the coordinating anions ofFeCo–N3O3@C, indicating thatFe atoms coordinate withN atoms inFeN4@C, and theCo–O3 part introducesd-FeN3@C and forms a Janus-likeFeNx–OxCo bimetallic site.
Figure 1 Research on carbon-based atomically dispersed metal catalysts
1. Synthesized and characterizedFeCo–N3O3@C catalyst
The authors developed aDAC synthesis strategy to synthesizeFeCo–N3O3@C Janus DAC nanosheets, and demonstrated the micro-morphology and local coordination environment of the catalyst through various characterization methods.
2. Tested the electrocatalytic performance ofFeCo–N3O3@C DAC.
The authors evaluated the electrocatalytic performance ofFeCo–N3O3@C DAC in an oxygen-saturated0.1 M KOH electrolyte using a typical three-electrode system, confirming its excellent bifunctional catalytic performance.
3. Analyzed the mechanism of electrocatalytic activity
The authors usedDFT calculations to deeply analyze the catalytic mechanisms ofORR andOER, and experimental characterizations confirmed that the strong synergistic interaction between theFe–N3 and Co–O3 parts can enhance the ORR and OER activity by reshaping the charge distribution of the dual-site active centers.
4. Investigated the performance of ZAB
The authors assembled a rechargeable aqueousZAB withFeCo–N3O3@C loaded on the cathode side in an air atmosphere, demonstrating excellent performance and outstanding stability compared to commercial batteries based onPt/C+RuO2.
1. Developed a feasible strategy to construct Janus bimetallic sites
The authors developed a feasible strategy to construct Janus bimetallic sites in efficient bifunctional catalysts, successfully synthesizing a carbon-based catalyst withFe-Co sites, and determined the structure of theFeCo-N3O3@C tetranuclear dimer catalyst through various characterization methods.
2. Achieved a bifunctional catalyst with excellent performance
The catalyst developed by the authors exhibits excellent bifunctional activity, outperforming reported single-atom counterparts. It was validated in a Zn-air battery with a power density of up to143 mW cm-2, and long-term durability exceeding200 hours. The high catalytic activity of theJanus FeCo–N3O3@C catalyst is attributed to the optimized filling of the 3d orbitals ofCo andFe atoms.
Synthesis and characterization of theFeCo–N3O3@C catalyst
The authors developed aDAC synthesis strategy that allows for the orderly assembly of two different functional components. The article presents the process of synthesizingFeCo–N3O3@C Janus DAC nanosheets through a three-step strategy. The loading ofFe andCo inFeCo–N3O3@C was determined to be1.16 and 0.97 wt% respectively by inductively coupled plasma atomic emission spectroscopy. SEM and TEM showed that all samples inherited a 2D hexagonal sheet morphology from the MOF precursor, with a thickness of about 30-100 nm. HRTEM, SAED, and PXRD did not observe any metal particles or clusters. HAADF-STEM indicated that nearly 100% of theFe atoms exist in single-atom form. After successfully introducing theCo-O3 part, a considerable proportion of bimetallic dimers appeared. The HAADF-STEM image ofFeCo–N3O3@C indicates that high-density heteronuclearFe–Co dual-atom sites were successfully obtained. Furthermore, the local coordination environment ofFe andCo atoms inFeCo–N3O3@C was studied using EXAFS spectroscopy, indicating thatFe species primarily exist in single-atom form inFeN4@C, and form metal-metal bonds after introducingCo–O3. XANES andXES further distinguished the coordinating anions ofFeCo–N3O3@C, indicating thatFe atoms coordinate withN atoms inFeN4@C, and theCo–O3 part introducesd-FeN3@C and forms a Janus-likeFeNx–OxCo bimetallic site.
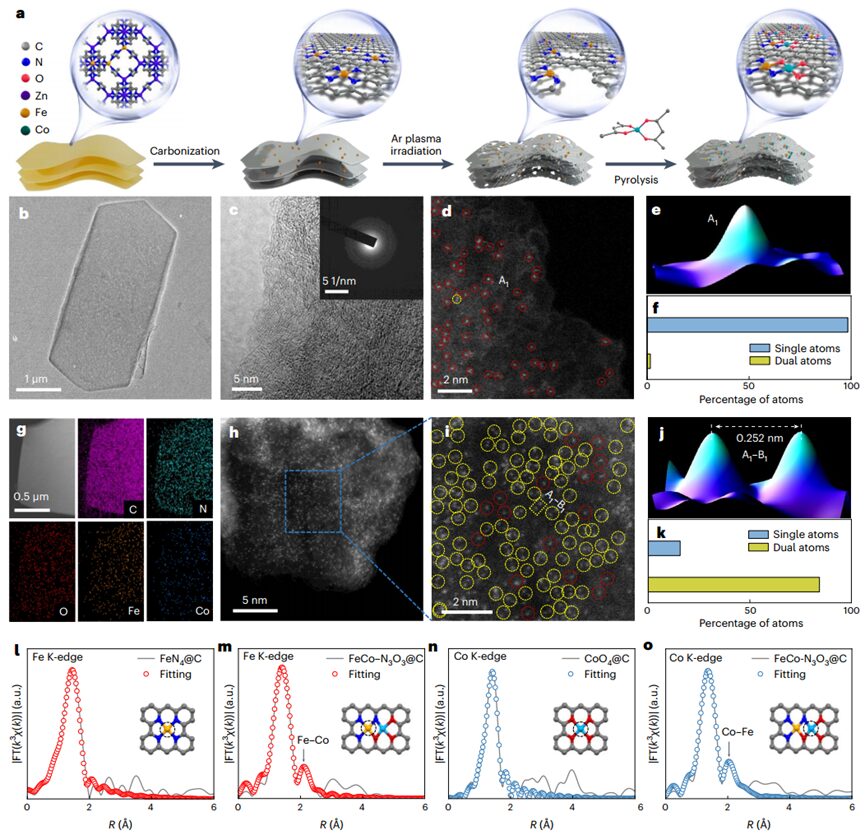 Figure 2 Synthesis scheme and morphological characterization ofFeCo–N3O3@C catalyst
Figure 3 Structural characterization ofFeCo–N3O3@ catalyst
Electrocatalytic Performance
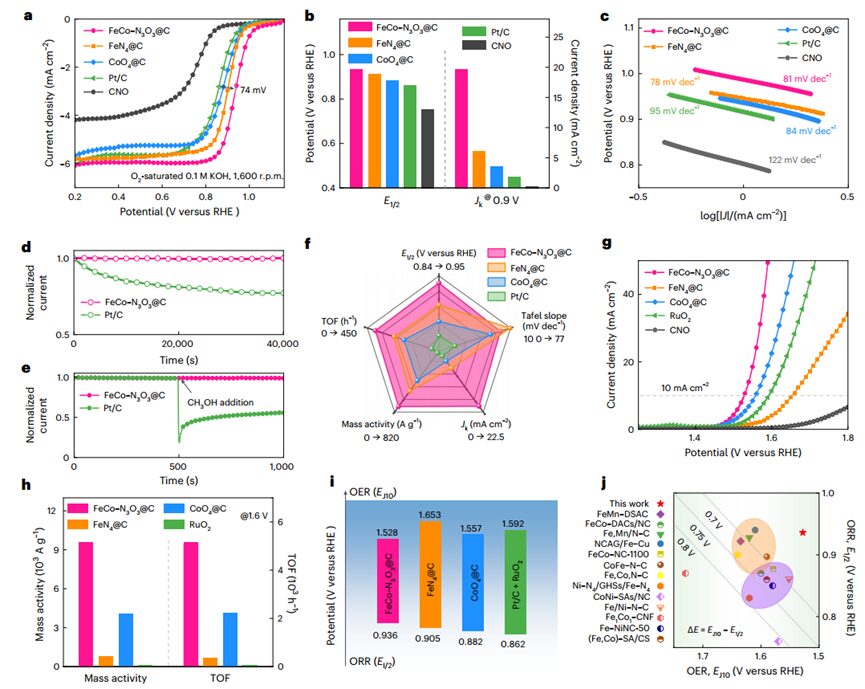
The authors evaluated the electrocatalytic performance ofFeCo–N3O3@C DAC inORR andOER using a typical three-electrode system in an oxygen-saturated0.1 M KOH electrolyte, and compared it withFeN4@C,CoO4@C,CNO, and commercialPt/C andRuO2.
The results showed thatFeCo–N3O3@C exhibited excellent catalytic capability forORR, with a low half-wave potential(E1/2=0.936 V), the highest kinetic current density (19.63 mA cm-2), and a low Tafel slope (81 mV dec-1). Additionally, the superior ORR activity ofFeCo–N3O3@C is further reflected in its higher mass activity and turnover frequency, indicating the synergistic effect of dual-atom sites in enhancingORR activity. TheFeCo–N3O3@C also exhibits high catalytic selectivity and stability. TheOER performance indicates thatFeCo-N3O3@C has the lowest overpotential and Tafel slope, demonstrating a high mass activity of9,558.3 A g-1 and a high TOF of5,100.4 h-1 at1.6 V. Moreover, the catalyst also exhibited excellent stability in a durability test lasting240 hours.
Figure 4 Catalytic performance ofFeCo–N3O3@C inORR andOER
Mechanism of Electrocatalytic Activity
The authors usedDFT calculations to deeply analyze the catalytic mechanisms ofORR andOER, revealing that there is a negative charge between theFe andCo atoms inFeCo–N3O3@C, indicating the formation ofFe–Co bonds and the redistribution of electrons in theDAC.The lower occupancy of theFe 3dz2 orbital can accelerate the transfer of electrons from oxygen to theFe site, promoting the adsorption and reduction of oxygen, thereby enhancing its activity inORR. Conversely, the higher occupancy of theCo 3dz2 orbital favors lowering the energy of electron transfer, as the electron affinity of theCo site is lower, thus facilitating theOER. Furthermore, the PDOS results indicate that the charge density ofFe andCo atoms near the Fermi level inFeCo–N3O3@C is greater than that inFeN4@C andCoO4@C, thus accelerating the transfer of electrons during the reaction process. Further in situSR-FTIR andXANES spectroscopy indicate that the strong synergistic interactions between theFe–N3 and Co–O3 parts can lower the energy barriers ofORR andOER by reshaping the charge distribution of the dual-site active centers, thereby enhancing the activity of bothORR andOER.
Figure 2 Synthesis scheme and morphological characterization ofFeCo–N3O3@C catalyst
Figure 3 Structural characterization ofFeCo–N3O3@ catalyst
Electrocatalytic Performance
The authors evaluated the electrocatalytic performance ofFeCo–N3O3@C DAC inORR andOER using a typical three-electrode system in an oxygen-saturated0.1 M KOH electrolyte, and compared it withFeN4@C,CoO4@C,CNO, and commercialPt/C andRuO2.
The results showed thatFeCo–N3O3@C exhibited excellent catalytic capability forORR, with a low half-wave potential(E1/2=0.936 V), the highest kinetic current density (19.63 mA cm-2), and a low Tafel slope (81 mV dec-1). Additionally, the superior ORR activity ofFeCo–N3O3@C is further reflected in its higher mass activity and turnover frequency, indicating the synergistic effect of dual-atom sites in enhancingORR activity. TheFeCo–N3O3@C also exhibits high catalytic selectivity and stability. TheOER performance indicates thatFeCo-N3O3@C has the lowest overpotential and Tafel slope, demonstrating a high mass activity of9,558.3 A g-1 and a high TOF of5,100.4 h-1 at1.6 V. Moreover, the catalyst also exhibited excellent stability in a durability test lasting240 hours.
Figure 4 Catalytic performance ofFeCo–N3O3@C inORR andOER
Mechanism of Electrocatalytic Activity
The authors usedDFT calculations to deeply analyze the catalytic mechanisms ofORR andOER, revealing that there is a negative charge between theFe andCo atoms inFeCo–N3O3@C, indicating the formation ofFe–Co bonds and the redistribution of electrons in theDAC.The lower occupancy of theFe 3dz2 orbital can accelerate the transfer of electrons from oxygen to theFe site, promoting the adsorption and reduction of oxygen, thereby enhancing its activity inORR. Conversely, the higher occupancy of theCo 3dz2 orbital favors lowering the energy of electron transfer, as the electron affinity of theCo site is lower, thus facilitating theOER. Furthermore, the PDOS results indicate that the charge density ofFe andCo atoms near the Fermi level inFeCo–N3O3@C is greater than that inFeN4@C andCoO4@C, thus accelerating the transfer of electrons during the reaction process. Further in situSR-FTIR andXANES spectroscopy indicate that the strong synergistic interactions between theFe–N3 and Co–O3 parts can lower the energy barriers ofORR andOER by reshaping the charge distribution of the dual-site active centers, thereby enhancing the activity of bothORR andOER.
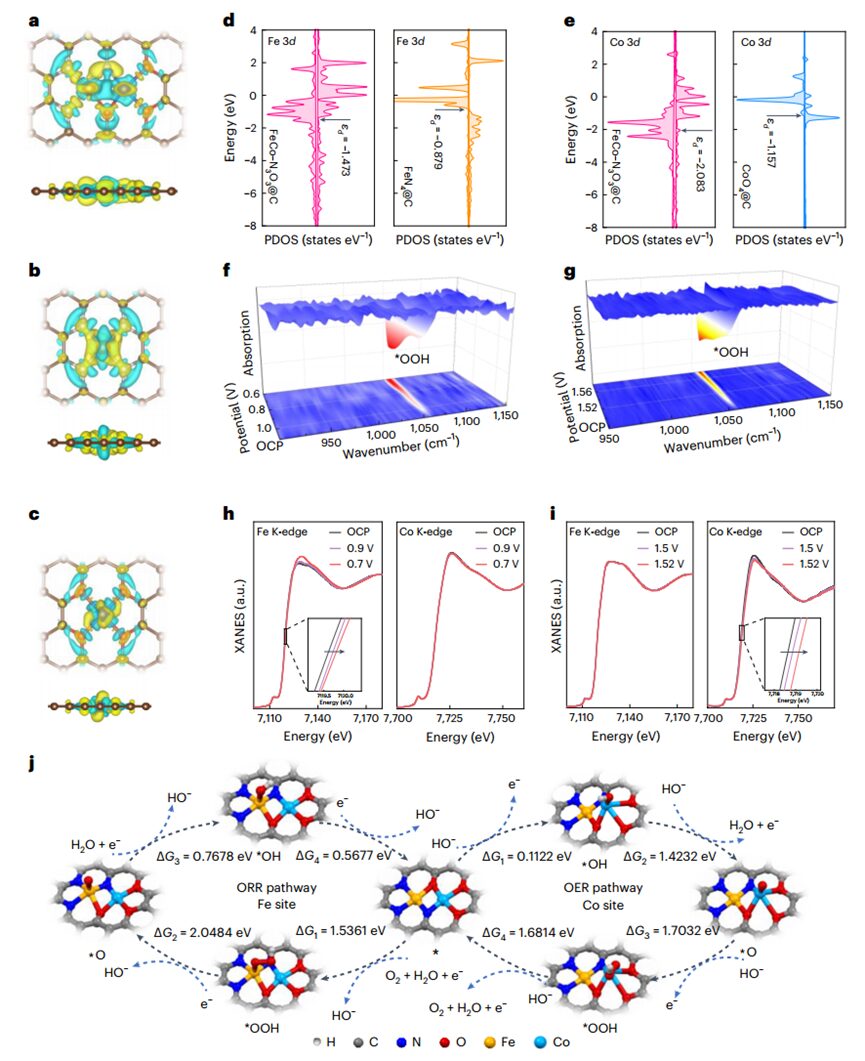 Figure 5 DFT calculations and in situSR-FTIR andXANES measurements
Given the excellent bifunctional activity and stability ofFeCo–N3O3@C inOER andORR, the authors assembled a rechargeable aqueousZAB loaded withFeCo–N3O3@C on the cathode side in an air atmosphere. Compared to commercial batteries based on Pt/C+RuO2, theZAB withFeCo–N3O3@C based air cathode exhibits higher open-circuit voltage (1.43 V), higher peak power density (143 mW cm−2), and higher specific capacity (787.2 mAh/g Zn). Additionally, at all current densities (1-10 mA cm-2), theZAB based onFeCo–N3O3@C exhibits extraordinary rate performance. Furthermore, three series-connectedZAB powered an LED scrolling display, confirming the potential application ofFeCo–N3O3@C in energy storage devices. Constant current cycling tests show that the battery based onFeCo–N3O3@C operates stably for over200 hours at10 mA cm-1. More importantly, PXRD, TEM, HAADF-STEM, and XANES and EXAFS spectroscopy indicate thatFeCo–N3O3@C maintains its original characteristics even after continuous charging/discharging cycles, demonstrating excellent durability and stability of the catalyst.
Figure 5 DFT calculations and in situSR-FTIR andXANES measurements
Given the excellent bifunctional activity and stability ofFeCo–N3O3@C inOER andORR, the authors assembled a rechargeable aqueousZAB loaded withFeCo–N3O3@C on the cathode side in an air atmosphere. Compared to commercial batteries based on Pt/C+RuO2, theZAB withFeCo–N3O3@C based air cathode exhibits higher open-circuit voltage (1.43 V), higher peak power density (143 mW cm−2), and higher specific capacity (787.2 mAh/g Zn). Additionally, at all current densities (1-10 mA cm-2), theZAB based onFeCo–N3O3@C exhibits extraordinary rate performance. Furthermore, three series-connectedZAB powered an LED scrolling display, confirming the potential application ofFeCo–N3O3@C in energy storage devices. Constant current cycling tests show that the battery based onFeCo–N3O3@C operates stably for over200 hours at10 mA cm-1. More importantly, PXRD, TEM, HAADF-STEM, and XANES and EXAFS spectroscopy indicate thatFeCo–N3O3@C maintains its original characteristics even after continuous charging/discharging cycles, demonstrating excellent durability and stability of the catalyst.
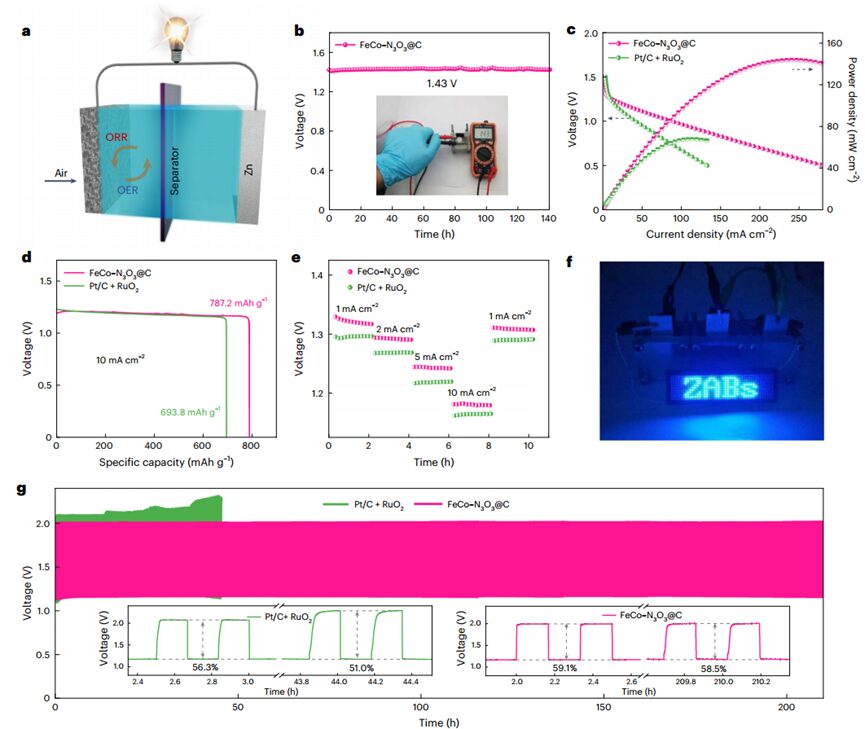 Figure 6 Performance ofZAB based onFeCo–N3O3@C andPt/C+RuO2.
In conclusion, the authors developed a simpleJanus FeCo–N3O3@C DAC synthesis strategy. Thanks to the unique configuration and strong interactions of theFeCo–N3O3 tetranuclear dimer, theJanus FeCo–N3O3@C catalyst demonstrates significantORR andOER activity and durability. Experimental and theoretical results indicate that charge transfer occurs between adjacentFe andCo atoms, and the synergistic effect ofFe–N3 andCo–O3 parts significantly reshapes the charge distribution of the metal active sites, optimizing the adsorption and desorption of reaction intermediates, effectively enhancing the bifunctionalORR/OER performance. TheFeCo–N3O3@C catalyst was successfully assembled inZAB, achieving a power density of up to143 mW cm−2, with charging/discharging cycle stability exceeding200 hours. This work provides new insights for the rational design of novel dual-atom sites on carbon surfaces to achieve high-performance electrocatalysts.
Tang, B., Zhou, Y., Ji, Q.et al. A Janus dual-atom catalyst for electrocatalytic oxygen reduction and evolution. Nat. Synth (2024).
https://doi.org/10.1038/s44160-024-00545-1
Electrocatalysis Discussion Group-1: 529627044
Photocatalysis Group-2: 927909706
Homogeneous Catalysis and Enzyme Catalysis Group-2: 929342001
Nano-Catalysis Group-1: 256363607
Porous Materials Group-2: 813094255
Theoretical Calculations Group-2: 863569400
Synchrotron Radiation丨Spherical Aberration Electron Microscopy丨FIB-TEM
In Situ XPS, In Situ XRD, In Situ Raman, In Situ FTIR
Figure 6 Performance ofZAB based onFeCo–N3O3@C andPt/C+RuO2.
In conclusion, the authors developed a simpleJanus FeCo–N3O3@C DAC synthesis strategy. Thanks to the unique configuration and strong interactions of theFeCo–N3O3 tetranuclear dimer, theJanus FeCo–N3O3@C catalyst demonstrates significantORR andOER activity and durability. Experimental and theoretical results indicate that charge transfer occurs between adjacentFe andCo atoms, and the synergistic effect ofFe–N3 andCo–O3 parts significantly reshapes the charge distribution of the metal active sites, optimizing the adsorption and desorption of reaction intermediates, effectively enhancing the bifunctionalORR/OER performance. TheFeCo–N3O3@C catalyst was successfully assembled inZAB, achieving a power density of up to143 mW cm−2, with charging/discharging cycle stability exceeding200 hours. This work provides new insights for the rational design of novel dual-atom sites on carbon surfaces to achieve high-performance electrocatalysts.
Tang, B., Zhou, Y., Ji, Q.et al. A Janus dual-atom catalyst for electrocatalytic oxygen reduction and evolution. Nat. Synth (2024).
https://doi.org/10.1038/s44160-024-00545-1
Electrocatalysis Discussion Group-1: 529627044
Photocatalysis Group-2: 927909706
Homogeneous Catalysis and Enzyme Catalysis Group-2: 929342001
Nano-Catalysis Group-1: 256363607
Porous Materials Group-2: 813094255
Theoretical Calculations Group-2: 863569400
Synchrotron Radiation丨Spherical Aberration Electron Microscopy丨FIB-TEM
In Situ XPS, In Situ XRD, In Situ Raman, In Situ FTIR