PET/CT has currently been recommended for imaging assessment at the initial diagnosis of multiple myeloma (MM) patients, as well as for evaluating the efficacy after treatment. In recent years, with the application of new drugs and autologous stem cell transplantation (ASCT), it has become possible for MM patients to pursue minimal residual disease (MRD) negativity. PET/CT can be combined with MRD detection methods such as next-generation flow cytometry or next-generation sequencing, providing a more accurate assessment of the MRD status post-treatment in MM patients. However, unlike lymphoma, the definition of complete metabolic response (CMR) in MM remains unclear. Researchers have used the DS (Deauville Scale) 5-point method of PET/CT to assess CMR after treatment in newly diagnosed MM (NDMM) patients, and this was recently published in the Journal of Clinical Oncology.
Research Background
18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) can accurately and sensitively detect bone disease in MM, especially extramedullary disease (EMD), and assess the metabolic activity of tumors. It evaluates treatment response by distinguishing between active and inactive lesions. Numerous previous studies have shown that even if patients achieve complete response (CR) after MM treatment, a positive lesion on PET/CT is associated with poor prognosis and short survival. With the continuous improvement of MM treatment efficacy, pursuing MRD negativity has become possible. Both domestic and international guidelines currently recommend PET/CT as a method for imaging MRD detection after MM treatment; patients who achieve MRD negativity via next-generation flow cytometry or next-generation sequencing, along with PET/CT, have better prognoses. However, the standards for CMR using PET/CT in MM remain unclear, lacking large sample prospective data analysis. The PET/CT DS 5-point method effectively improves the precision of efficacy evaluation standards in lymphoma patients, but whether it applies to NDMM patients is still uncertain.
Recently, researchers analyzed patients with baseline and post-treatment PET/CT data from independent European, randomized, phase III clinical studies on NDMM (IFM/DFCI2009 and EMN02/HO95 trials), aiming to determine the CMR standards for PET/CT post-treatment in NDMM.
Research Methods
The IFM/DFCI2009 trial prospectively evaluated the efficacy differences between eight cycles of lenalidomide, bortezomib, and dexamethasone (RVd) versus RVd combined with ASCT, followed by lenalidomide maintenance therapy. The EMN02/HO95 trial prospectively compared the efficacy differences between single ASCT versus double ASCT versus bortezomib-based intensified treatment after induction therapy in NDMM patients.
This study analyzed data from patients in the IFM/DFCI2009 and EMN02/HO95 trials with complete PET/CT examination results both at baseline and prior to maintenance treatment (PM), including the metabolic status of bone marrow (BM), focal lesions (FL; quantity and metabolic status), and EMD (site, quantity, and metabolic status). The primary aim of the study was to describe the metabolic status of BM (BM; BM score [BMS]) and FL (FL score [FS]) using the PET/CT DS, and to analyze their impact on clinical outcomes in MM patients through univariate and multivariate analyses. Furthermore, it aimed to standardize PET/CT assessments and define the CMR standards for PET/CT post-treatment (i.e., PET/CT-MRD definition).
Research Results
The study included 228 patients (134 from the IFM/DFCI2009 trial and 94 from the EMN02/HO95 trial). The median age of patients was 59 years; 15.8% and 11.5% of patients were staged as III according to ISS and R-ISS, respectively. 14% of patients had high-risk cytogenetic abnormalities detected by FISH [t(4;14), del(17p), and/or t(14;16)]. The median follow-up time was 62.9 months. According to the trial design, 57% of patients were randomly assigned to the transplant group, while 43% were assigned to the bortezomib-based intensified treatment group (54% in IFM/DFCI2009 and 24% in EMN02/HO95). The best PM response rates were similar in both trials (CR 36.7%; VGPR 82.5%).
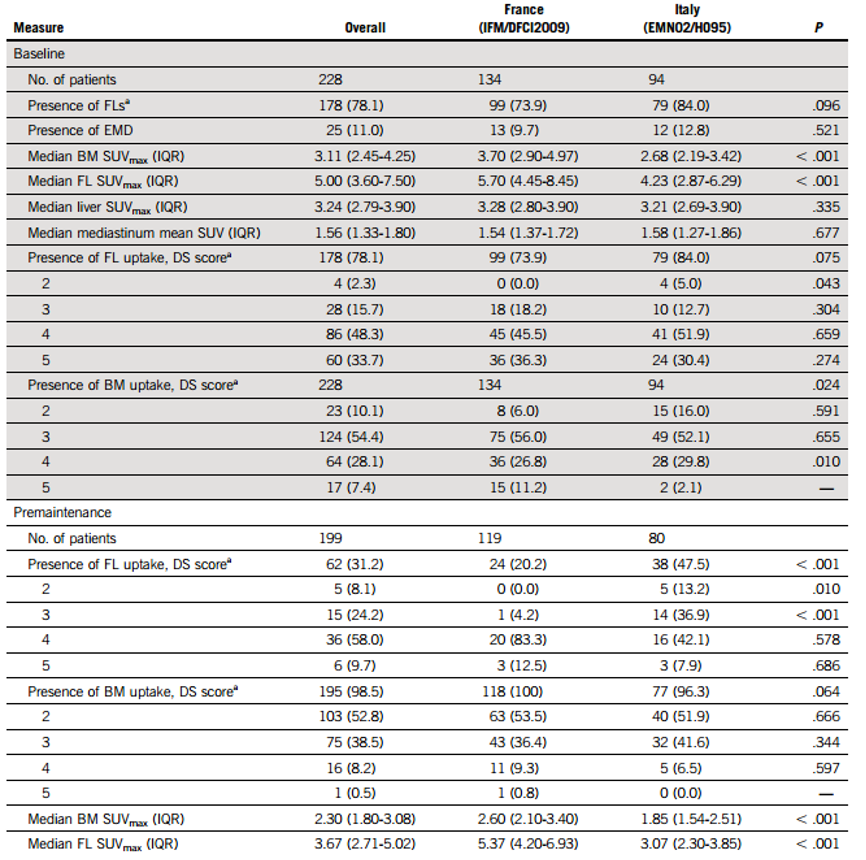
At baseline, 78% of patients had FL-positive lesions (11% had EMD), with a median SUVmax of 5, and 80% of patients had FS≥4; all patients showed diffuse uptake in BM, with a median SUVmax of 3.11, and 35.5% of patients had BMS≥4. The baseline median SUVmax for FL and BM in the IFM/DFCI2009 trial was slightly higher than that in the EMN02/HO95 trial (see Table 1). The distributions of median SUVmax in the mediastinal blood pool (MBP) and hepatic blood pool, as well as FS, BMS, and EMD (11% of patients), were similar between the two trials.
At PM, 31% of patients still had FL-positive lesions, with a median SUVmax of 3.67; EMD persisted in 2% of patients. The uptake scores for residual FL were 2 points (8.1%), 3 points (24.2%), 4 points (58%), or 5 points (9.7%). 98% of patients had residual diffuse uptake in BM, but significantly lower than baseline levels (52.8% had BMS of 2 points, 38.5% had BMS of 3 points, 8.2% had BMS of 4 points, and 0.5% had BMS of 5 points); 85% of patients had BMS drop from baseline ≥4 to <4 at PM, with a median SUVmax of 2.3. The PM median SUVmax for FL and BM in the IFM/DFCI2009 trial was also slightly higher than that in the EMN02/HO95 trial. Overall, 53.5% and 71.2% of patients had FS and BMS <3 points, while 79% and 91.4% had FS and BMS <4 points. About 20% of patients had baseline BMS and/or FS <4 points, but ≥4 points at PM.
Table 1: PET/CT Examination Results at Baseline and PM for Patients
 To verify the correlation of each DS score with patient PFS and OS, and to determine the optimal clinically relevant PET/CT positive and negative cutoff values post-treatment. Researchers performed univariate prognostic analysis on all different DS scores for FL and BM at PM; results showed that patients with FS and BMS <4 had longer PFS [ (FS <4 vs ≥4: median PFS 40 vs 26.6 months, P=0.0307); (BMS <4 vs ≥4: median PFS 44.9 vs 26.6 months, P=0.028)] and OS [(FS <4 vs ≥4: estimated 60-month OS rate 77.7% vs 64.1%, P=0.0276); (BMS <4 vs ≥4: estimated 60-month OS rate 76.7% vs 52.1%, P=0.029)] (Table 2 and Figure 1). The DS score standard of FS ≤3 was associated with longer PFS and OS, but BMS was not, possibly because the reactive changes in BM are usually related to treatment. The DS scores of 4 for FL and BM were associated with patient PFS and OS. Therefore, researchers concluded that a DS score of 4 is the optimal cutoff for achieving CMR in PET/CT examination post-treatment for MM.
To verify the correlation of each DS score with patient PFS and OS, and to determine the optimal clinically relevant PET/CT positive and negative cutoff values post-treatment. Researchers performed univariate prognostic analysis on all different DS scores for FL and BM at PM; results showed that patients with FS and BMS <4 had longer PFS [ (FS <4 vs ≥4: median PFS 40 vs 26.6 months, P=0.0307); (BMS <4 vs ≥4: median PFS 44.9 vs 26.6 months, P=0.028)] and OS [(FS <4 vs ≥4: estimated 60-month OS rate 77.7% vs 64.1%, P=0.0276); (BMS <4 vs ≥4: estimated 60-month OS rate 76.7% vs 52.1%, P=0.029)] (Table 2 and Figure 1). The DS score standard of FS ≤3 was associated with longer PFS and OS, but BMS was not, possibly because the reactive changes in BM are usually related to treatment. The DS scores of 4 for FL and BM were associated with patient PFS and OS. Therefore, researchers concluded that a DS score of 4 is the optimal cutoff for achieving CMR in PET/CT examination post-treatment for MM.
Table 2: Univariate Analysis Results of PET/CT DS Scores at PM and Patient PFS and OS.
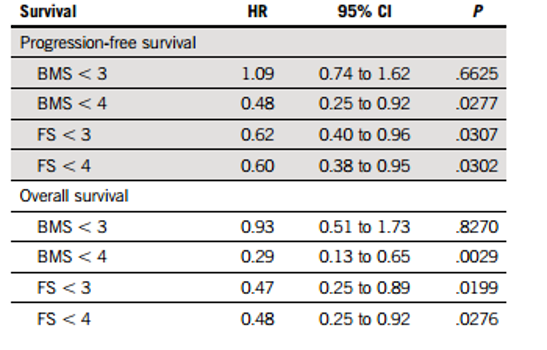
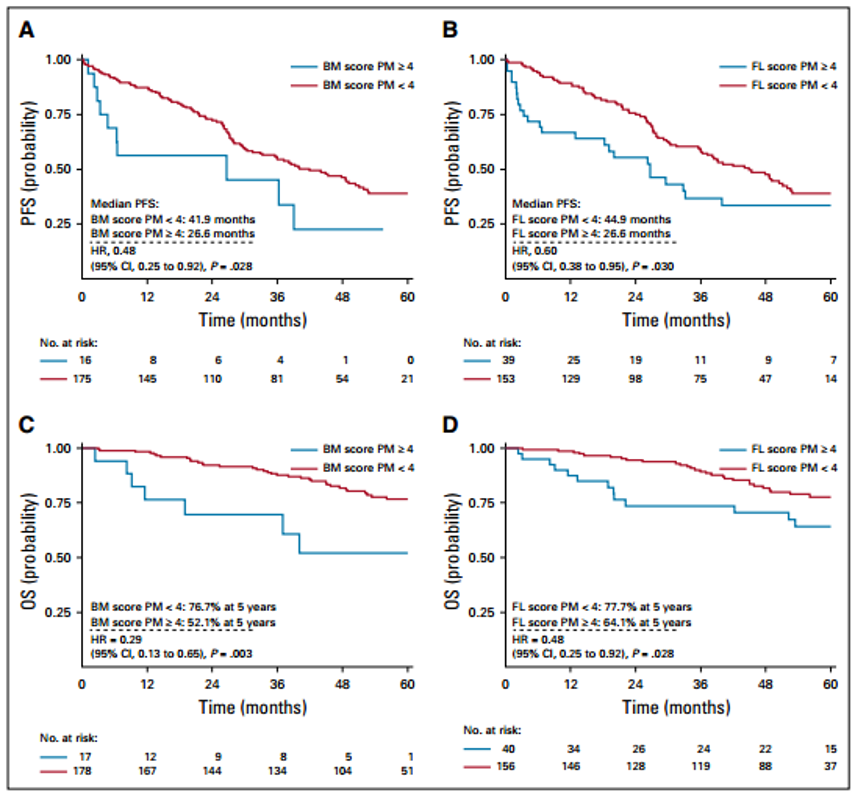
Figure 1: Relationship Between PET/CT DS Scores at PM and Patient PFS and OS: A/B, BM and FL Scores with PFS Relationship; C/D, BM and FL Scores with OS Relationship.
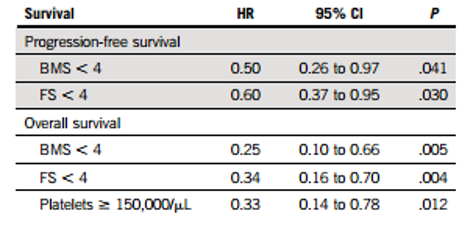
Cox multivariate analysis results showed that FS and BMS <4 were independent prognostic factors for longer PFS (FS: hazard ratio-HR, 0.60; 95% confidence interval-CI, 0.37-0.95; P=0.03; BMS: HR, 0.50; 95% CI, 0.26-0.97; P=0.041) and OS (FS: HR, 0.36; 95% CI, 0.17-0.74; P=0.05; BMS: HR, 0.24; 95% CI, 0.09-0.63; P=0.004). Multivariate analysis results consistently showed that FS <4 was associated with longer PFS and OS in the subgroup of patients included in the EMN02/HO95 trial.
Table 3: Cox Multivariate Analysis Results of PET/CT DS Scores at PM and Patient PFS and OS.
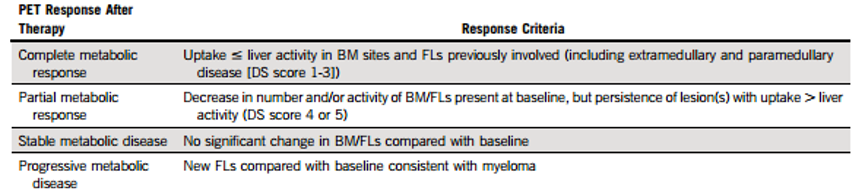
Based on the results of this study, the authors recommend using FDG uptake values of lesions including BM and FL (EMD and paraneoplastic diseases) ≤ hepatic background (i.e., DS score of 1-3 points) as the standard for achieving CMR in PET/CT post-treatment for MM (see Table 4).
Table 4: Suggested Improvements to PET/CT Efficacy Evaluation Standards Based on DS Score for MM Patients Post-Treatment.
Research Conclusion
In NDMM patients, PET/CT examination post-treatment showing FL and BM FDG uptake below the hepatic blood pool is an independent predictor of good PFS and OS, which can serve as the standard for PET/CT CMR; confirming the value of the PET/CT DS standard for efficacy evaluation in MM.
Elena Zamagni, Cristina Nanni, Luca Dozza, et al. Standardization of 18 F-FDG-PET/CT According to Deauville Criteria for Metabolic Complete Response Definition in Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2021 Jan 10;39(2):116-125.