In the 2021 National College Entrance Examination, both the National A卷 and Shandong卷 tested s-p-d hybridization. This type of hybridization may not typically be taught by teachers, and students may not learn it. Therefore, whether it is appropriate to test this knowledge in the college entrance examination is debatable. However, since it has been tested, it is best for us to be prepared. If students are tested without any study, they will find it difficult to adapt. This time, I will share the types and judgments of s-p-d hybridization.
First, let’s take a look at the 2021 college entrance examination situation:
2021 National A卷
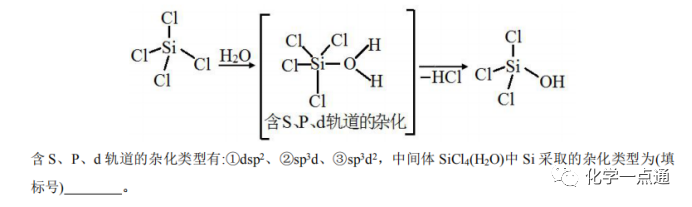
The 2021 Shandong卷 also examined the hybridization method of Xe atom in XeF2.
Next, let’s briefly discuss the types of s-p-d hybridization. Common s-p-d hybridizations include:dsp2, sp2d, dsp3, sp3d, d2sp3, sp3d2 and a few other situations. Note that the difference between dsp3 and sp3d lies in the principal quantum numbers of the participating d orbitals. The former involves hybridization of the n-level sp orbitals with the (n-1) level d orbitals, while the latter involves hybridization of the n-level sp orbitals with the n-level d orbitals. The hybridization results are the same, and the components of the hybrid orbitals do not differ much. In many cases, no distinction is made. So how can we make a distinction if needed?
For p-block elements, the energy of ns and np orbitals is quite close to that of nd orbitals, so p-block elements utilize s-p-d hybridization. For transition elements, the energy of ns and np orbitals is also relatively close to that of nd or (n-1) d orbitals, and which hybridization to use depends on which energy is closer. Therefore, some transition elements adopt s-p-d hybridization (forming outer orbital complexes), while others adopt d-s-p hybridization (forming inner orbital complexes). In other words, main group elements will not exhibit inner orbital d-s-p hybridization; they will only adopt s-p-d hybridization. So how can we determine which hybridization method to use for transition elements? Please see the screenshot below:
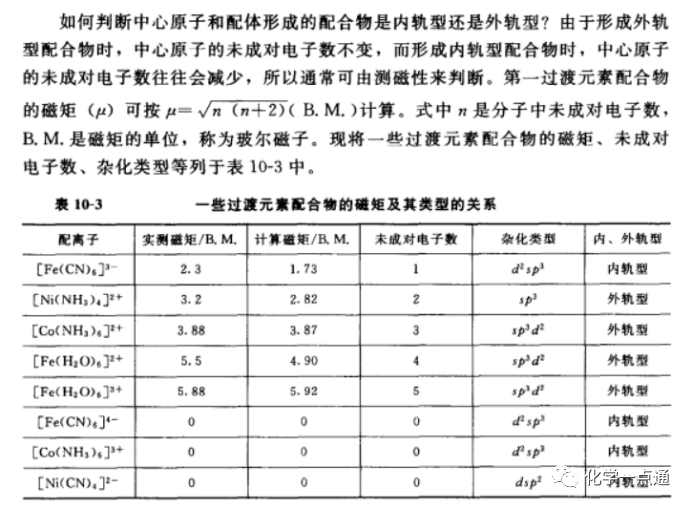
From the image, we can see that a magnetic moment of 0 indicates an inner orbital type, while a magnetic moment greater than 0 indicates an outer orbital type. We can also determine based on the number of unpaired electrons; those with unpaired electrons are outer orbital types, while those without unpaired electrons are inner orbital types. However, it should be noted that this unpaired electron situation is after hybridization, as the hybridization process of transition elements generally involves electron rearrangement. Therefore, we cannot judge based on the unpaired electrons of the atom or ion. For example, Fe2+ has a configuration of 3d6, and according to Hund’s rule, it has 4 unpaired electrons. But in reality, when it forms [Fe(CN)]6]2+, its 6 d electrons rearrange to empty two d orbitals, resulting in all 6 d electrons being paired. This is represented in the following diagram: 
Regarding the judgment of inner and outer orbital complexes, it is necessary to learn about crystal field theory and other knowledge. I think it is not necessary for us in high school to complicate things. From this year’s college entrance examination questions, this knowledge point belongs to a “borderline” topic. For example, the Shandong卷 examined the hybridization method of the Xe atom in XeF2, and the options provided were: A, sp; B, sp2; C, sp3; D, sp3d. Clearly, according to the valence shell electron pair repulsion theory, the central atom Xe‘s valence shell electron pairs are: (8+2)/2=5 pairs, so A, B, and C are obviously incorrect, so we can only choose D. Although the National A卷 had options for inner orbital d-s-p hybridization: ①dsp2; ②sp3d; ③sp3d2; it is easy to rule out option ①, as Si does not have d orbitals in its outer layer, so it cannot adopt d-s-p hybridization, making it easy to eliminate option ①. Then, based on the structural formula, it is relatively easy to choose option ②.
Teaching suggestions: In teaching, I believe we should briefly introduce students to the differences between d-s-p and s-p-d and provide basic rules. The p-block elements can only adopt s-p-d outer orbital type, while transition elements can be either s-p-d type or d-s-p type but will not be tested. According to the valence shell electron pair repulsion theory, if the central atom has 5 valence electron pairs, the p-block element will adopt sp3d hybridization; if the central atom has 6 valence electron pairs, the p-block element will adopt sp3d2 hybridization.
Previous Article Links