▲ Click the above blue text to follow CellPress Cell Science ▲


Life Science
Life science


Coupling CRISPR/Cas and Argonaute (Ago) programmable nucleases with isothermal amplification technologies effectively addresses the limitations of isothermal amplification technologies, such as RPA and LAMP, which suffer from poor specificity and difficulty in multiplex detection, providing new solutions for point-of-care testing (POCT). However, due to differences in reaction conditions and component interactions, amplification and nuclease reactions often need to be performed in steps, and opening the lid during the operation can easily lead to aerosol contamination of the amplification products, while also increasing operational complexity. How to achieve amplification-nuclease compatible “one-pot” detection has become a key bottleneck issue for the clinical application of programmable nuclease detection technologies. Recently, Professor Feng Yan and Researcher Liu Qian from the School of Life Science and Technology at Shanghai Jiao Tong University published a review titled “One-pot diagnostic methods based on CRISPR/Cas and Argonaute nucleases: strategies and perspectives” in the journal Trends in Biotechnology by Cell Press. This paper systematically reviews the challenges faced by the “one-pot” molecular diagnostic technology that couples CRISPR/Cas and Argonaute programmable nucleases with nucleic acid amplification, the methods and strategies for existing amplification-nuclease compatible reactions, and prospects for the future development of programmable nuclease detection technologies.


Interested in publishing your research paper or review article in Trends in Biotechnology? Please scan to submit your paper proposal (presubmission inquiry).
1. Microfluidic Chip Technology
Physical isolation is the most direct method to achieve “one-pot” CRISPR detection, commonly achieved by using special isolation media to fix amplification and cleavage reactions in two chambers, with manual operation to mix the cleavage system and amplification products after amplification. However, this approach still faces challenges such as reagent fixation stability and operational complexity. Microfluidic chips integrate multiple reaction chambers into a single reaction carrier, driven by external forces to achieve physical separation of amplification and nuclease reactions, enhancing sensitivity, multiplexing, and automation levels. For example, the DISCoVER system, which utilizes gravity to drive amplification products into the Cas reaction system, has achieved high sensitivity and multiplex detection of SARS-CoV-2. Pressure-driven microfluidic biosensors (FA-MB) and centrifugal microfluidic chips provide efficient fluid handling and new methods for multiplex “one-pot” detection. Although the development of microfluidic chips is complex and costly, advancements in 3D printing and reduced material costs indicate that there is still significant room for improvement in this method.
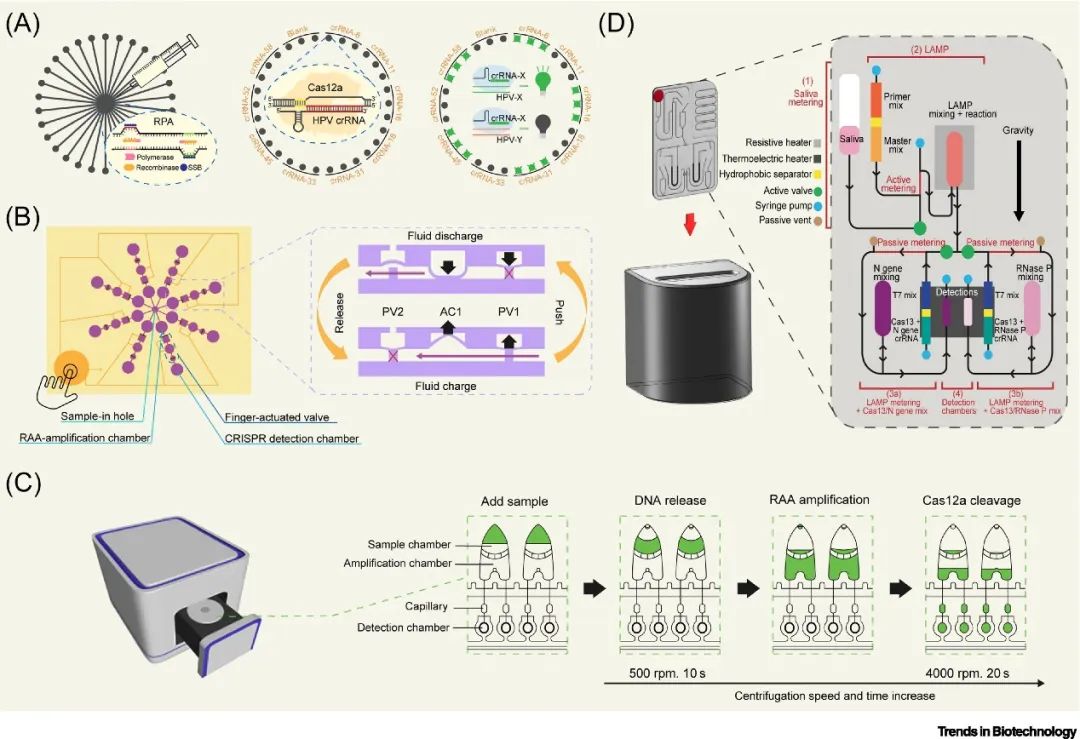
▲ Figure 1. Physical separation of amplification and nuclease reactions using microfluidic chips
2. Component Optimization
Optimizing the components of the reaction system is also an important way to achieve “one-pot” CRISPR detection. By adjusting the concentrations of primers, crRNA, Cas proteins, dNTPs, metal ions, or adding additional auxiliary factors such as RNase H and RNase inhibitors in the reaction buffer, the balance between amplification and Cas cleavage efficiency can be regulated, improving sensitivity and detection efficiency. For example, STOPCovid V2 optimized the required components of the Cas cleavage system and systematically screened buffer components to achieve high sensitivity detection of SARS-CoV-2. Additionally, choosing amplification systems that are more compatible with Cas protein reaction conditions, such as transcription-mediated amplification (TMA) and rolling circle amplification (RCA), can also enable effective “one-pot” detection. Standardized and streamlined experimental design (DOE) can maximize optimization efficiency. Although component optimization does not require specialized consumables and instrument design, the optimization process remains complex due to the varying efficiencies of amplification and cleavage for different targets, and the generalizability of the optimized system still requires further validation with a larger number of targets.
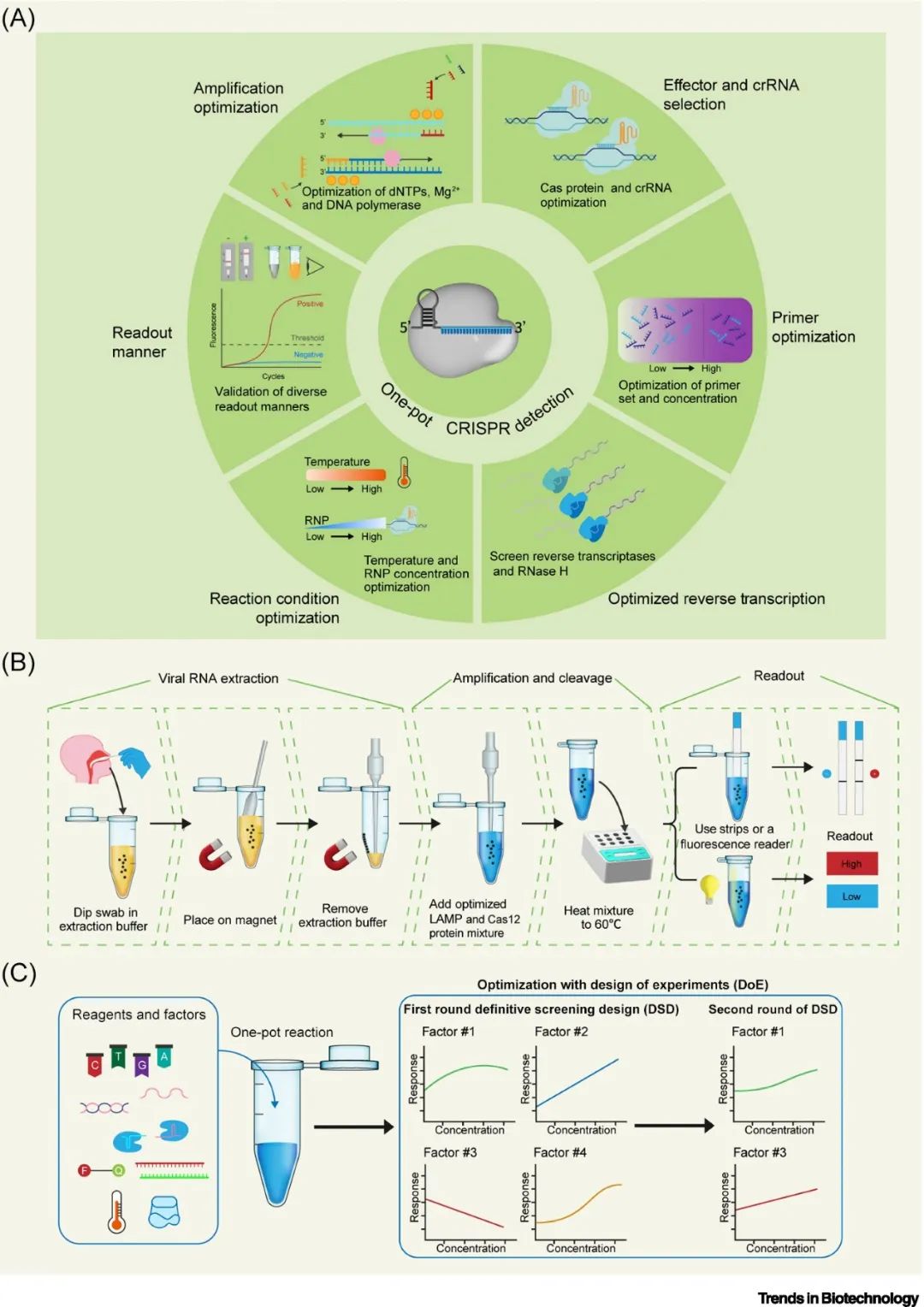
▲ Figure 2. Achieving “one-pot” detection through optimization of reaction components
3. Mining and Engineering of Cas Proteins
Mining and engineering of Cas proteins can yield novel Cas proteins with higher tolerance to amplification systems, particularly enhancing their thermal stability, which is an important direction for improving Cas proteins. Novel thermally stable Cas proteins (such as AacCas12b and BrCas12b variants) can withstand higher temperatures, making them compatible with the reaction conditions of LAMP amplification technology. Additionally, constructing protein mutants using artificial intelligence and machine learning can enhance thermal stability and reduce non-specific template DNA binding capacity, improving sensitivity and robustness of detection. With the continuous elucidation of the structure-function relationship of proteins and the development of high-throughput protein screening technologies, directed evolution to modify the activity of Cas proteins may become an important avenue for improving the efficiency of “one-pot” detection.
4. Modification of Guide RNAs
Modifying guide crRNA to regulate Cas12a activity is another method to achieve “one-pot” detection. Light-controlled blocking nucleic acids, suboptimal PAM, and no PAM designs help to address the problem of nucleic acids being prematurely cleaved by Cas12a, reducing the interference of Cas proteins on primers and templates in the amplification system, thereby improving overall efficiency and sensitivity.
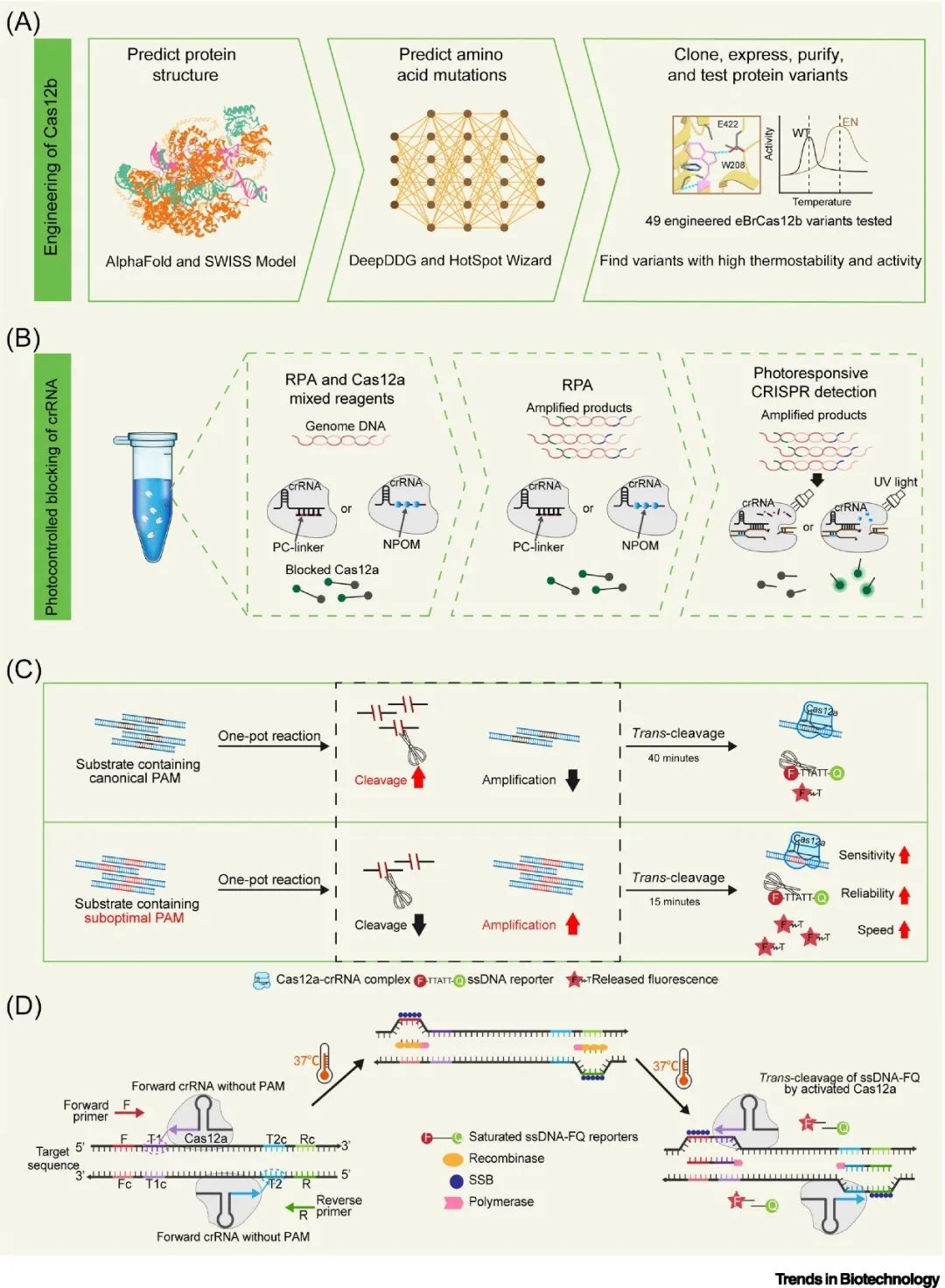
▲ Figure 3. Achieving “one-pot” detection through modifications of Cas proteins and crRNA
5. Ago-Based “One-Pot” Detection Technology
Compared to CRISPR technology, Ago-based detection technology is still in its early stages, but it has shown significant application potential due to its characteristics of short ssDNA guide strands and single-enzyme target detection. Utilizing the unique properties of thermophilic Ago proteins, combined with custom consumables for physical isolation, the SPOT system has achieved temperature-controlled “one-pot” detection, while another OPTIMAL system has developed heat-activated immobilized Ago enzymes to enable stepwise initiation of amplification and cleavage reactions within conventional reaction tubes. These innovations provide economical, efficient, and robust solutions for nucleic acid detection and are expected to expand into more POCT application scenarios.
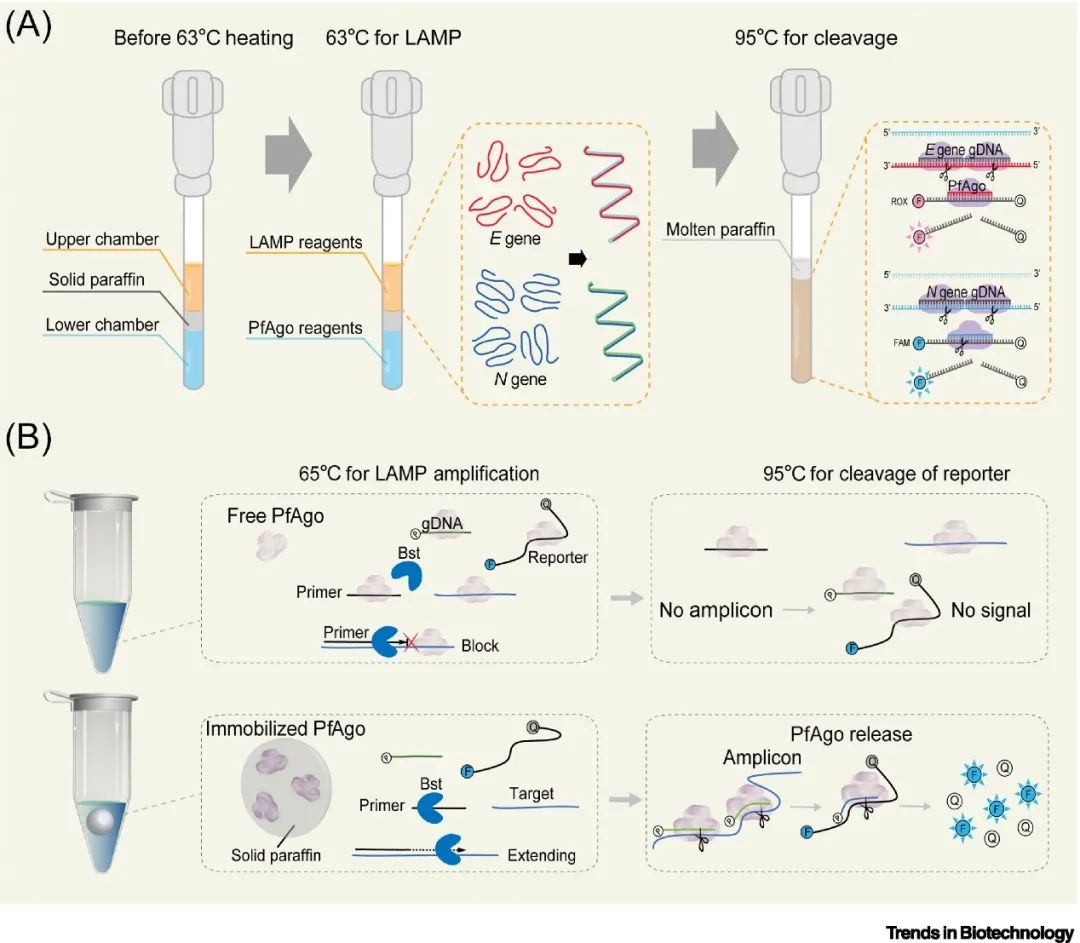
▲ Figure 4. Heat-activated “one-pot” detection based on thermophilic Argonaute
Summary and Outlook
“One-pot” detection technologies based on CRISPR and Ago demonstrate broad application prospects in infectious disease diagnosis, environmental monitoring, and agriculture. Despite their immense potential, challenges such as specialized equipment consumables, optimization complexity, and generalizability validation still need to be addressed. Future research will focus on the discovery of novel enzymes, enhancement of enzyme formulation controllability, and miniaturization and automation of supporting equipment. The development of artificial intelligence, protein engineering, and electrochemical biosensors will further improve development cycles, enzymatic performance, and usability, driving the evolution of “one-pot” detection technologies and developing more efficient, sensitive, and user-friendly next-generation molecular diagnostic tools in the field of point-of-care testing.
References for this article (scroll to view)
1. Chen, J.S. et al. (2018) CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360, 436-439
2. Gootenberg, J.S. et al. (2018) Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 360, 439-444
3. Wang, R. et al. (2021) opvCRISPR: One-pot visual RT-LAMP-CRISPR platform for SARS-cov-2 detection. Biosens Bioelectron 172, 112766
4. Xu, Z. et al (2022) Microfluidic space coding for multiplexed nucleic acid detection via CRISPR-Cas12a and recombinase polymerase amplification. Nat Commun 13, 6480
5. Joung, J. et al. (2020) Detection of SARS-CoV-2 with SHERLOCK one-pot testing. N Engl J Med 383, 1492-1494
6. Yan, H. et al. (2023) A one-pot isothermal Cas12-based assay for the sensitive detection of microRNAs. Nat Biomed Eng 7, 1583-1601
7. Mahas, A. et al. (2022) Characterization of a thermostable Cas13 enzyme for one-pot detection of SARS-CoV-2. Proc Natl Acad Sci U S A 119, e2118260119
8. Tong, X. et al. (2024) Fast and sensitive CRISPR detection by minimized interference of target amplification. Nat Chem Biol, doi: 10.1038/s41589-023-01534-9
9. Yang, J. et al. (2023) Engineered LwaCas13a with enhanced collateral activity for nucleic acid detection. Nat Chem Biol 19, 45-54
10. Hu, M. et al. (2022) Photocontrolled crRNA activation enables robust CRISPR-Cas12a diagnostics. Proc Natl Acad Sci U S A 119, e2202034119
11. Lu, S. et al. (2022) Fast and sensitive detection of SARS-CoV-2 RNA using suboptimal protospacer adjacent motifs for Cas12a. Nat Biomed Eng 6, 286-297
12. Ding, X. et al. (2020) Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat Commun 11, 4711
13. Ye, X. et al. (2022) Argonaute-integrated isothermal amplification for rapid, portable, multiplex detection of SARS-CoV-2 and influenza viruses. Biosens Bioelectron 207, 114169
14. Xun, G. et al. (2021) A rapid, accurate, scalable, and portable testing system for COVID-19 diagnosis. Nat Commun 12, 2905
15. Ye, X. et al. (2024) Thermal activation of argonaute nuclease enables one-pot multiplex detection of viruses. Sensors and Actuators B-Chemical 409, 135587


Related paper information

Related research published in Cell Press
In the journal Trends in Biotechnology,
Click “Read the original text” or scan the QR code below to view the paper

▌Paper Title:
One-pot diagnostic methods based on CRISPR/Cas and Argonaute nucleases: strategies and perspectives
▌Paper URL:
https://www.sciencedirect.com/science/article/pii/S0167779924001562
▌DOI:
https://doi.org/10.1016/j.tibtech.2024.06.009

▲ Long press to recognize the QR code to read the paper
Recommended Reading

Trends in Biotechnology Review | Pickering Emulsion Oil-Water Interface: Microenvironment for Living Cell Biocatalysis

▲ Long press to recognize the QR code to follow Cell Science
