Focused and dedicated, stepping onto the international stage
Written by|Little Medicine Monster
The latest generation of anti-HER-2 ADC drug developed by Hengrui, SHR-A1811, is often jokingly referred to as the domestic alternative to DS-8201 due to its similar structure. However, strength will eliminate all rumors.
Results from early clinical studies released by AACR this year show that, compared to DS-8201, SHR-A1811 demonstrates comparable efficacy, holding its own numerically; more importantly, SHR-A1811 seems to have superior safety, with a lower incidence of interstitial pneumonia, laying a solid foundation for combination use, long-term treatment, and frontline advancement.
Looking at the development speed of Hengrui through the SHR-A1811 project, it started from preclinical research on 2020.06.25, entered clinical phase I on 2020.10.19, just a year after the approval of the first indication for DS-8201 globally (2019.12.20); on 2021.06.04, it entered clinical phase II; on 2022.08.10, just two days after the approval of the second indication for DS-8201, SHR-A1811 announced its entry into clinical phase III, keeping pace efficiently, showcasing the kingly demeanor of a Chinese big pharma.
Considering that DS-8201 was only approved domestically earlier this year and has not yet been commercially launched, alongside Hengrui’s local advantages (R&D advantages + commercial advantages); coupled with Hengrui’s deep cultivation in the breast cancer field, Pyrotinib is another self-developed anti-HER-2 small molecule new drug by the company, with indications spanning HER-2 positive breast cancer, from neoadjuvant to advanced lines. In the future, with the support of SHR-A1811, the two will undoubtedly become the golden partners on Hengrui’s path against HER-2, sweeping the domestic HER-2 positive solid tumor market and using this as a base to stride towards the international arena. Hengrui has never forgotten innovation and internationalization.
Overview of the Clinical Layout of SHR-A1811
As of 2023.04.16, the Citeline database shows that SHR-A1811 has registered a total of 18 clinical studies: 2 phase III, 5 phase II, 6 phase I/II, 5 phase I. (Intensively conducted from 2020-2023)
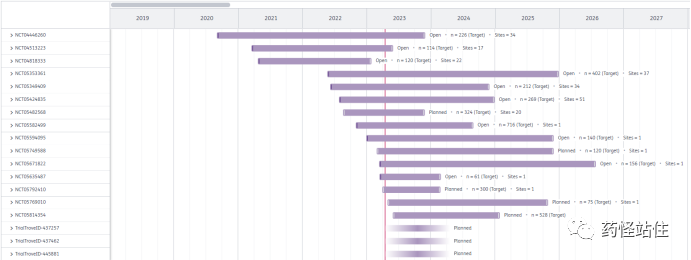
SHR-A1811-III-306 (NCT05814354) is a randomized, parallel-controlled, multicenter, open-label phase III study exploring the efficacy and safety of SHR-A1811 for HER2 low-expressing recurrent or metastatic breast cancer compared to investigator’s choice chemotherapy, with the primary endpoint being BICR assessed PFS. Expected to start on 2023.05.30, with primary endpoint results anticipated on 2025.01.30. This study benchmarks against DS-8201‘s DB04.
SHR-A1811-III-301 (NCT05424835) is a randomized, parallel-controlled, multicenter, open-label phase III study exploring the efficacy and safety of SHR-A1811 compared to “Pyrotinib + Capecitabine” for advanced HER-2 positive breast cancer previously treated with “Trastuzumab + Paclitaxel”, with the primary endpoint being BICR assessed PFS. The study started on 2022.07.29, with primary endpoint results expected on 2024.12.31. This study benchmarks against DS-8201‘s DB03, differing in that the international second-line standard treatment for advanced HER-2 positive breast cancer is TDM-1 (Roche’s Herceptin), so the control group for DB03 is TDM-1. However, due to accessibility and other reasons, “Pyrotinib + Capecitabine” is the standard treatment in China, receiving the highest recommendation in the 2022 breast cancer CSCO guidelines, classified as level I recommendation, with class IA evidence (higher than TDM-1‘s class 1B evidence), thus the control group for 301 is “Pyrotinib + Capecitabine”, aligning with domestic clinical practice, making this a study belonging to China.
HNCH-MBC12 (NCT05769010) is a prospective, open-label phase II study exploring the efficacy and safety of SHR-A1811 for HER-2 positive late-stage breast cancer with brain metastasis.
SCHBCC-N046 (NCT05749588) is an open-label phase II study exploring the feasibility of precision medicine strategies for recurrent triple-negative breast cancer based on molecular typing in a single-center platform.
MUKDEN 07 (NCT05635487) is a single-arm phase II study exploring the efficacy and safety of “SHR-A1811 + Pyrotinib” as neoadjuvant therapy for resectable HER-2 positive breast cancer.
SCHBCC-N041 (NCT05594095) is a prospective, single-center, open-label phase II study exploring the feasibility of precision flat treatment strategies for HR+/HER2- based on SNP typing.
SHR-A1811-II-201 (NCT05349409) is a dose-exploration, expansion phase II study exploring the efficacy and safety of SHR-A1811 combined with Fuzolapali (a PARP inhibitor developed by Hengrui) for HER-2 expressing advanced solid tumors.
SHR-A1811-II-202 (NCT05353361) is an open-label, multicenter Ib/II phase study exploring the efficacy and safety of SHR-A1811 combined with Pyrotinib/Patuzumab/SHR-1316/Abraxane for HER-2 positive breast cancer.
SHR-A1811-103 (NCT04818333) is an I/II phase study exploring the safety, tolerability, PK, and efficacy of SHR-A1811 for HER-2 overexpressing, amplifying, or mutated non-small cell lung cancer.
FASCINATE-N (NCT05582499) is an I/II phase study, part of the precision medicine series of studies from the Fudan Breast Cancer Center.
SHR-A1811-II-203 (NCT05482568) is an Ib/II phase study exploring the safety, tolerability, PK, and efficacy of “SHR-A1811 + Pyrotinib + SHR-1316″ for advanced non-small cell lung cancer expressing HER-2.
SHR-A1811-206 (NCT05792410) is an open-label, multicenter Ib/II phase study exploring the efficacy and safety of SHR-A1811 combined with Darlisilib (a CDK4/6 inhibitor developed by Hengrui), Fulvestrant, Bevacizumab, and Letrozole for HER-2 low-expressing advanced or metastatic breast cancer.
SHR-A1811-Ib/II-205 (NCT05671822) is an I/II phase study exploring the efficacy and safety of “SHR-A1811 + chemotherapy ± immunotherapy” for HER-2 positive advanced gastric cancer/gastroesophageal junction adenocarcinoma.
TrialTroveID-445881 is an I phase study exploring the efficacy and safety of SHR-A1811 for HER-2 positive gastric cancer/gastroesophageal junction adenocarcinoma.
TrialTroveID-437462 is an I phase study exploring the efficacy and safety of SHR-A1811 combined with Pyrotinib for HER-2 abnormal non-small cell lung cancer.
TrialTroveID-437257 is an I phase study exploring the efficacy and safety of SHR-A1811 combined with Atezolizumab (a PD-L1 antibody developed by Hengrui) for HER-2 abnormal non-small cell lung cancer.
SHR-A1811-I-102 (NCT04513223) is an I phase study exploring the safety, tolerability, PK, and efficacy of SHR-A1811 for HER-2 expressing gastric cancer, gastroesophageal junction adenocarcinoma, and colorectal cancer.
SHR-A1811-I-101 (NCT04446260) is a multicenter, open-label I phase study exploring the safety, tolerability, PK, and efficacy of SHR-A1811 for HER-2 expressing or mutated advanced solid tumors.
As we organize, we find that SHR-A1811 can be fully paired with Hengrui’s existing pipeline: including but not limited to Abraxane, Atezolizumab, Darlisilib, Pyrotinib, and Fuzolapali. Excluding Hengrui, no other company currently holds such a comprehensive asset portfolio.
Hengrui, recognized as the number one innovative drug company in China, may not be perfect yet, but is striving to improve; please give it some time.