Click the above “CAIVD” to follow us
In 2018, the National Medical Products Administration released the “Catalog of Medical Devices Exempt from Clinical Trials” (hereinafter referred to as the new “Exemption Catalog”).
The new “Exemption Catalog” includes a total of 1248 medical devices exempt from clinical trials, divided into two parts: “Medical Device Products” and “In Vitro Diagnostic Reagent Products”, covering 855 medical device products and 393 in vitro diagnostic reagent products, respectively. Compared to the previous three batches of exemption catalogs, 84 new medical device products and 277 new in vitro diagnostic reagent products have been added. The new “Exemption Catalog” is largely consistent with the new “Classification Catalog” to facilitate applicants in better identifying products and integrating all previously published exemption catalogs for easier reference.
The release of the new “Exemption Catalog” once again expands the scope of medical devices exempt from clinical trials, further aligning China’s requirements for clinical trials of medical devices with international standards; it reduces the clinical trial requirements for products with high maturity and low risk, alleviating the burden on enterprises, allowing them to focus more on product R&D and quality improvement; it also promotes a clinical evaluation approach based on product risk, optimizes clinical trials and review resources, and directs valuable resources towards urgently needed and innovative medical device products, facilitating the rapid market entry of safe, effective, and controllable products to meet the growing demand for medical devices from the public.
Therefore, I have compiled a list of 50 in vitro diagnostic instruments exempt from clinical trials and 393 in vitro diagnostic reagents, as shown in the images below (click to view the large image):
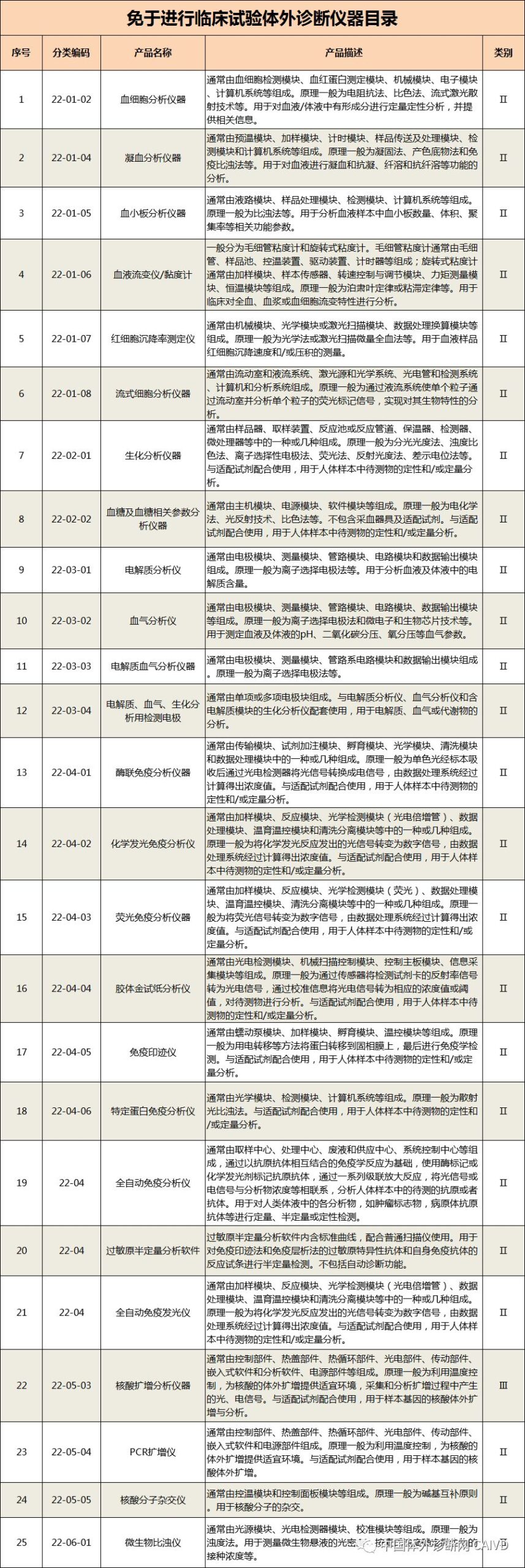
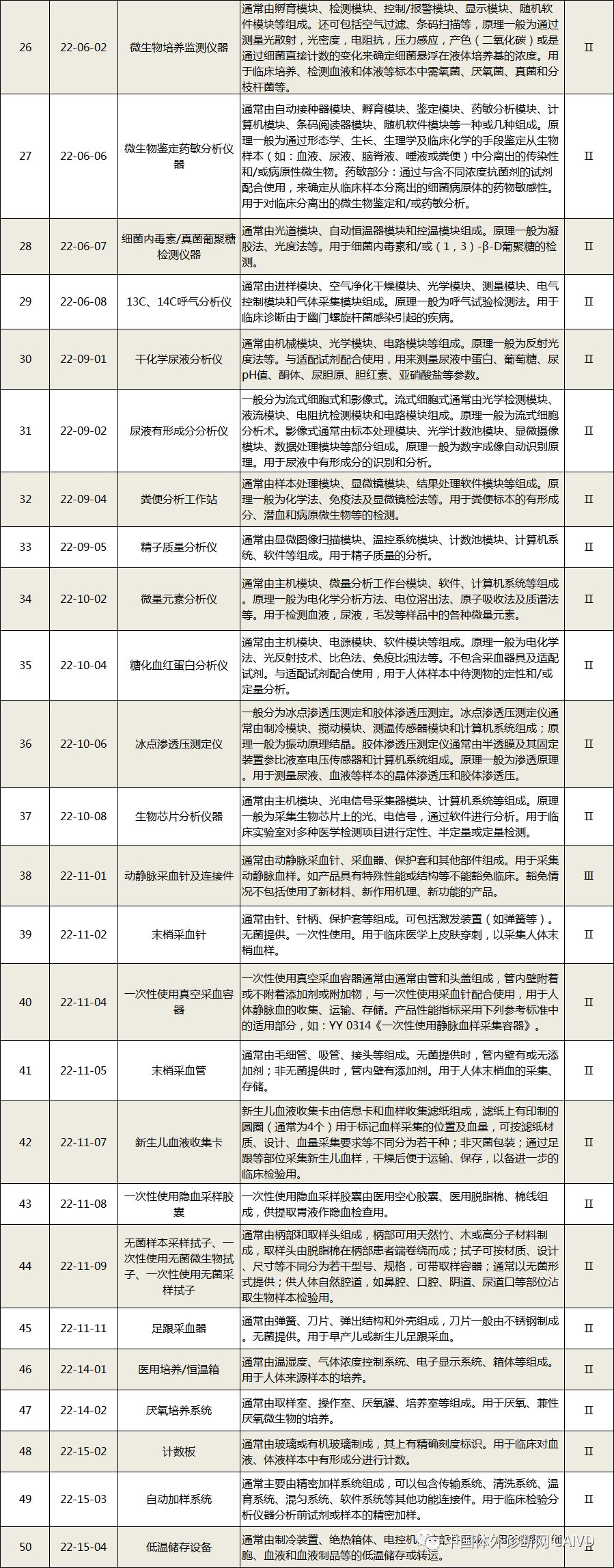
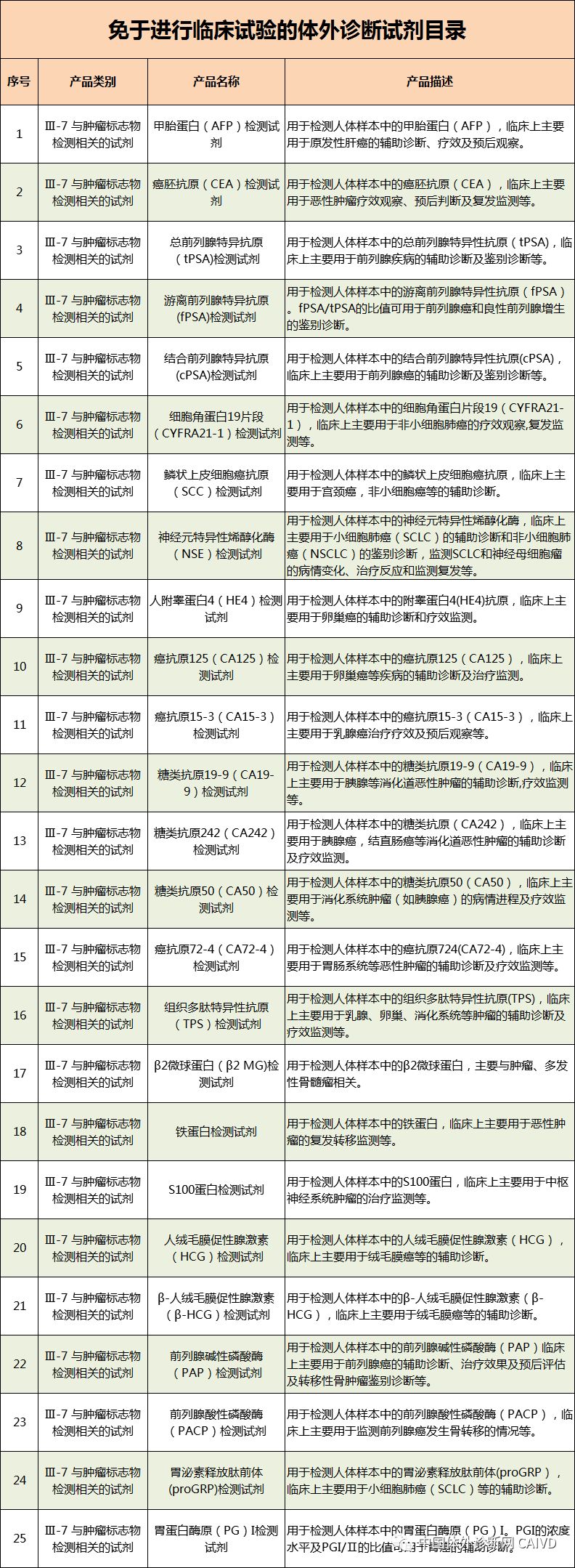
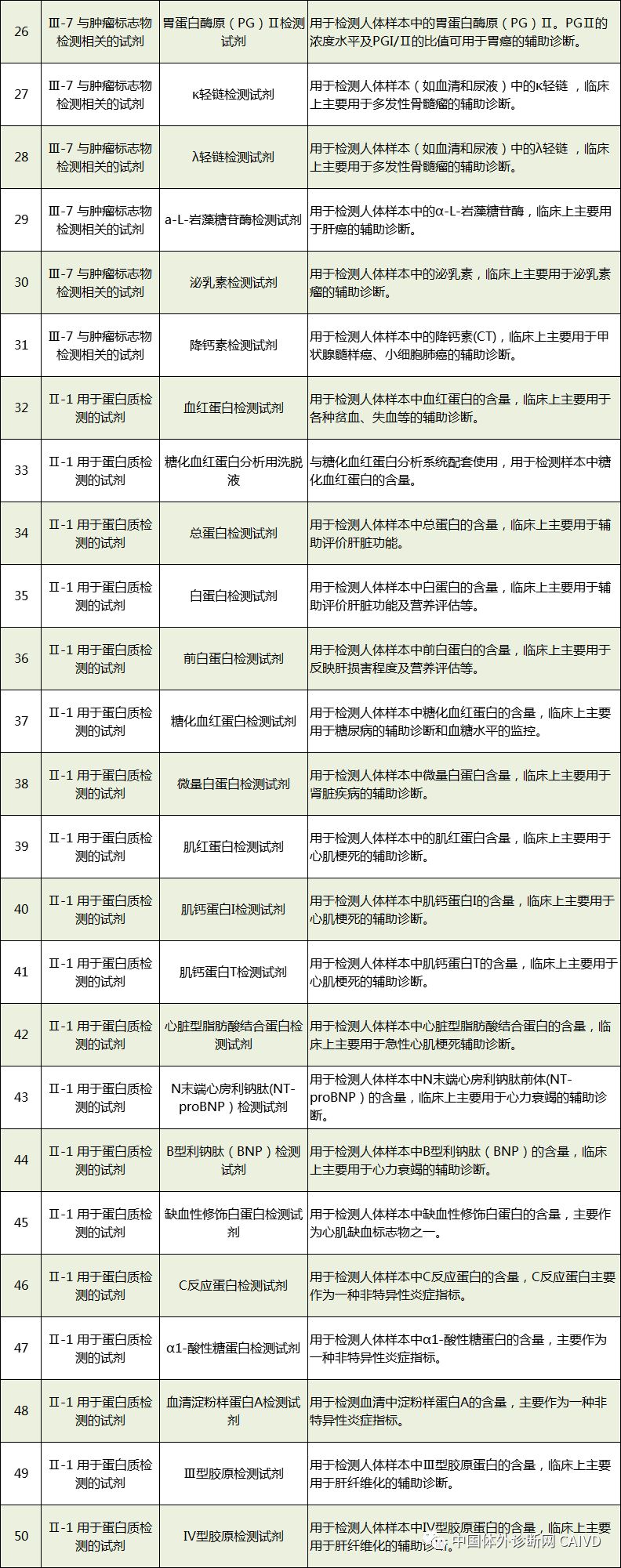
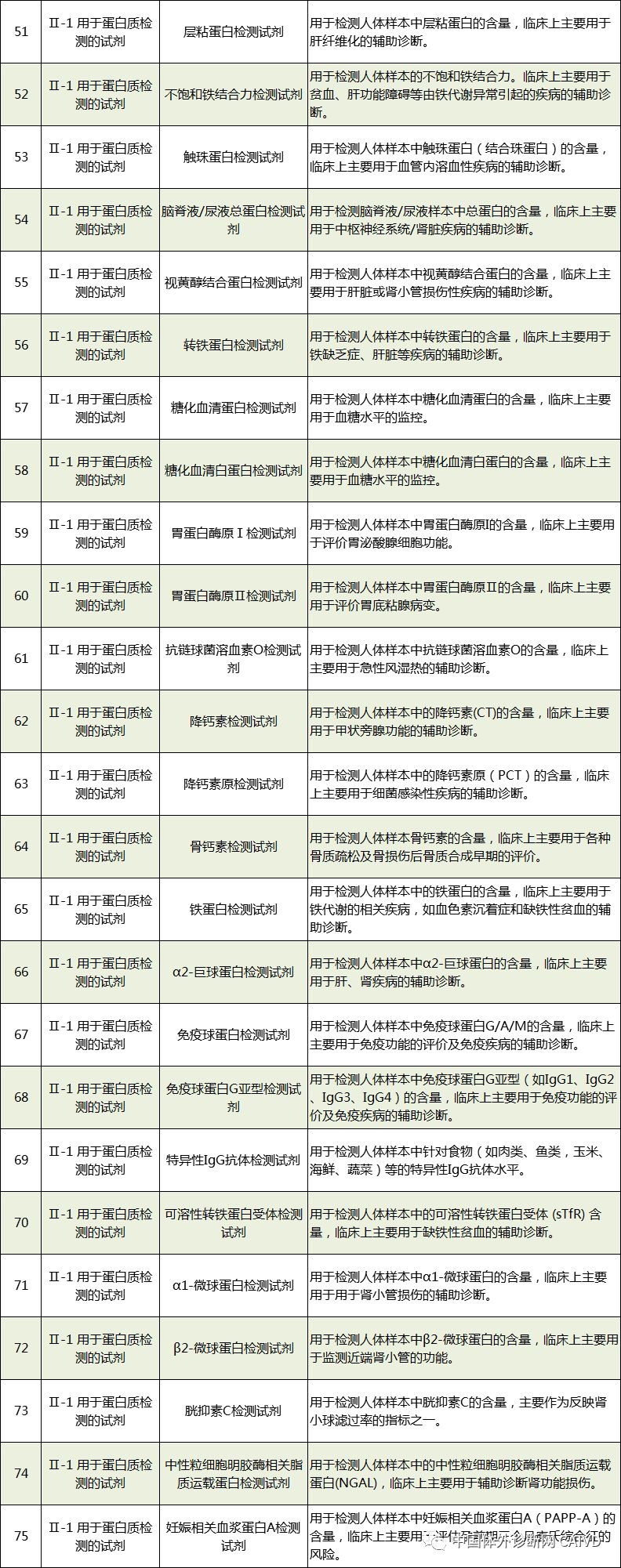
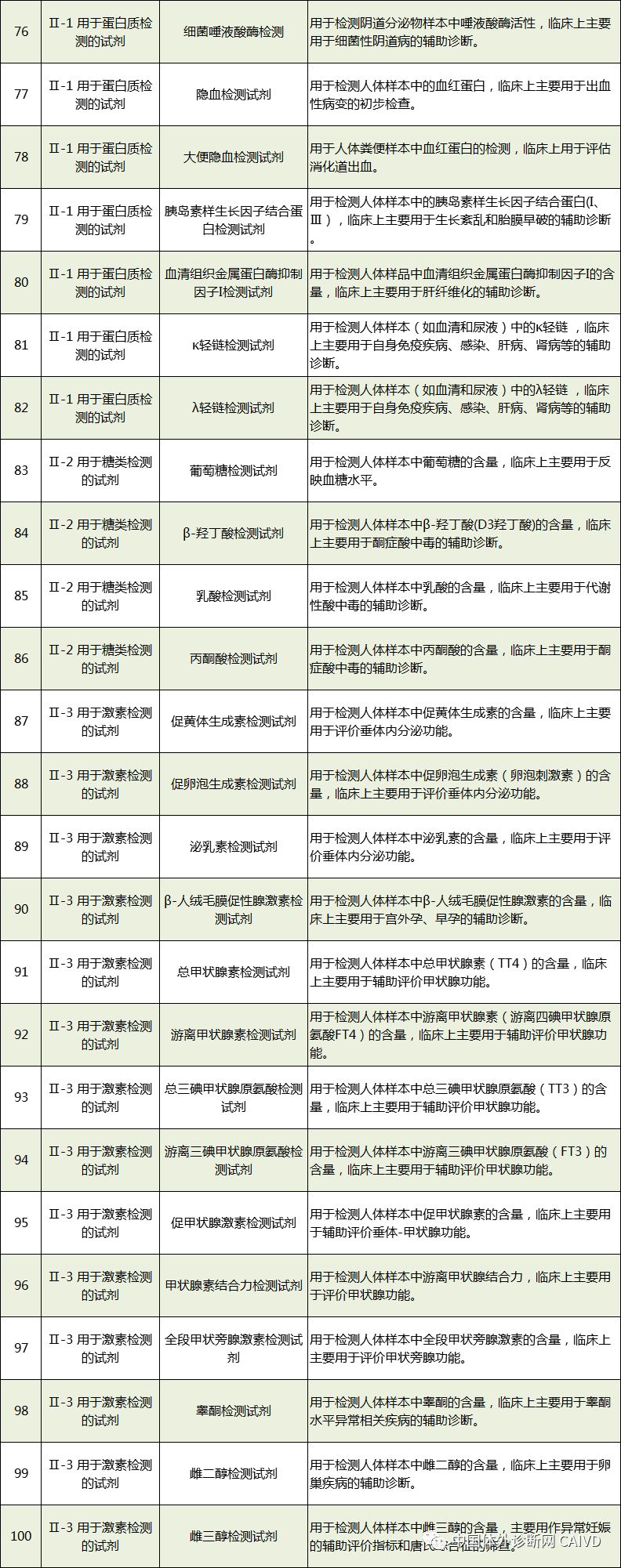
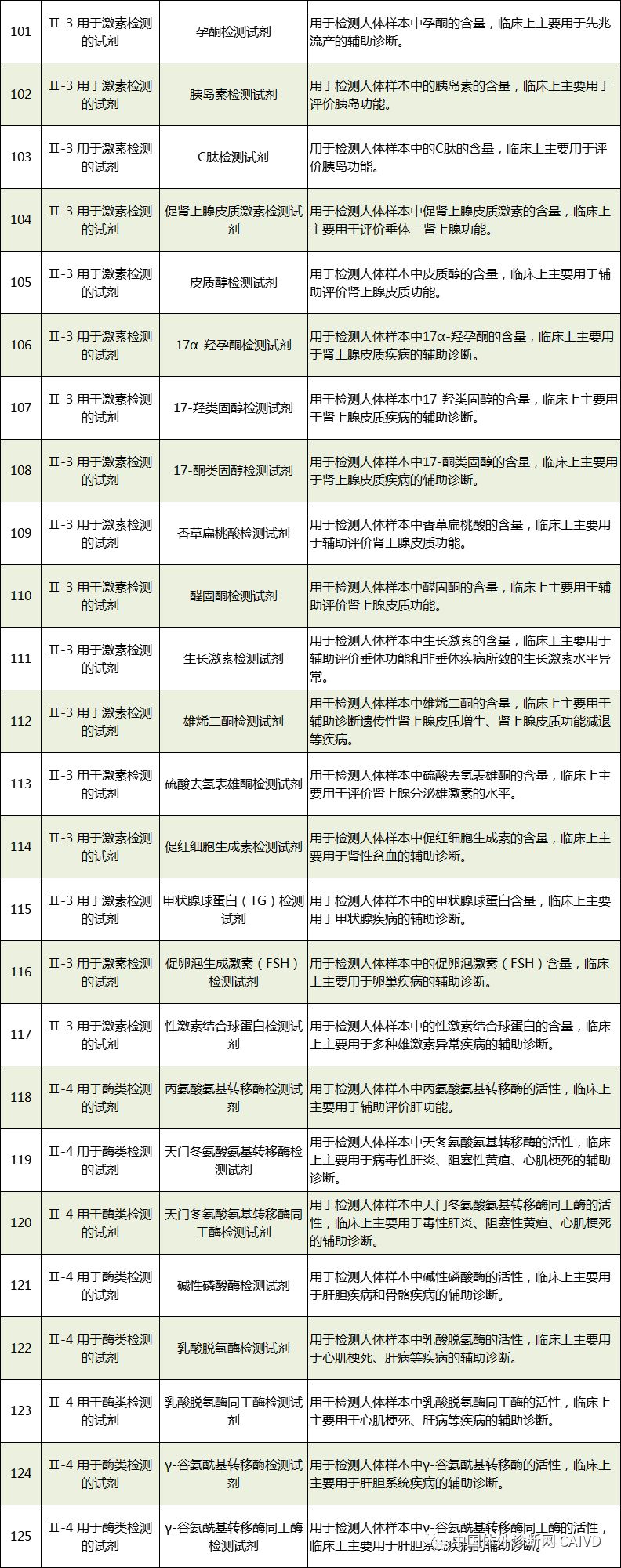
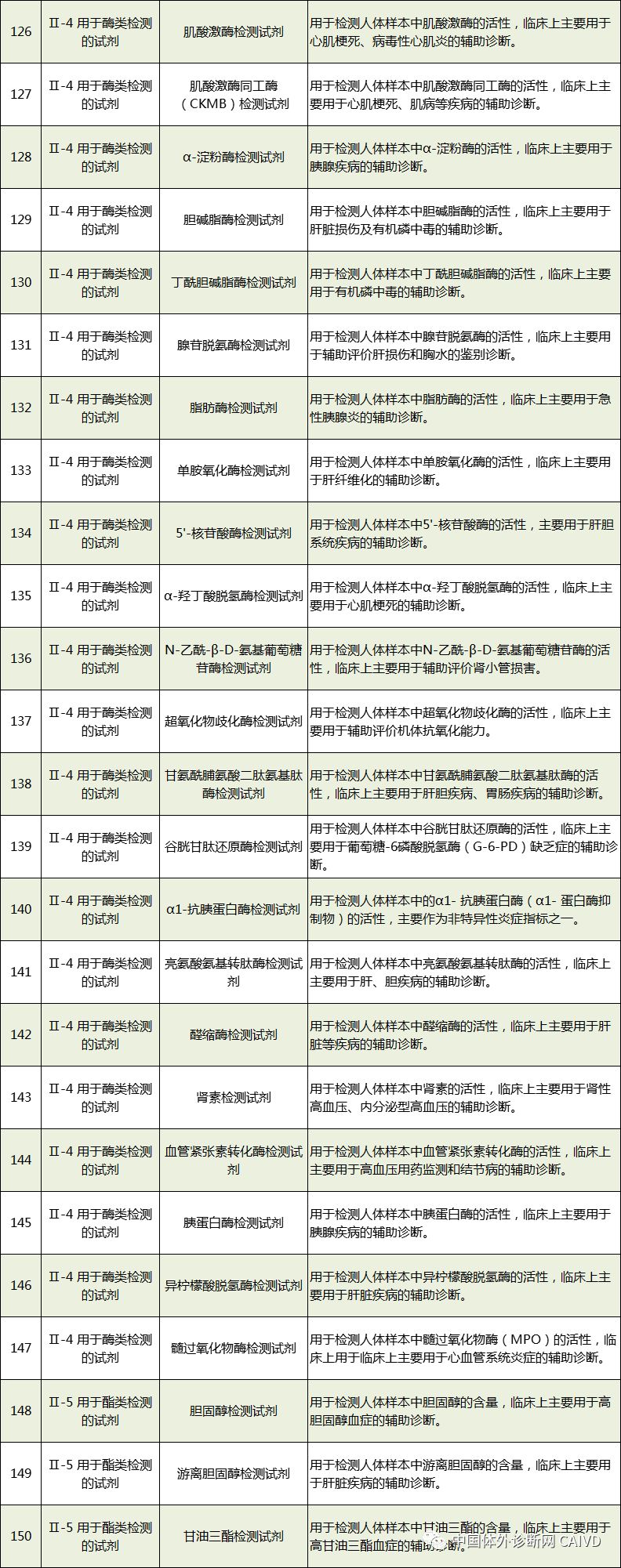
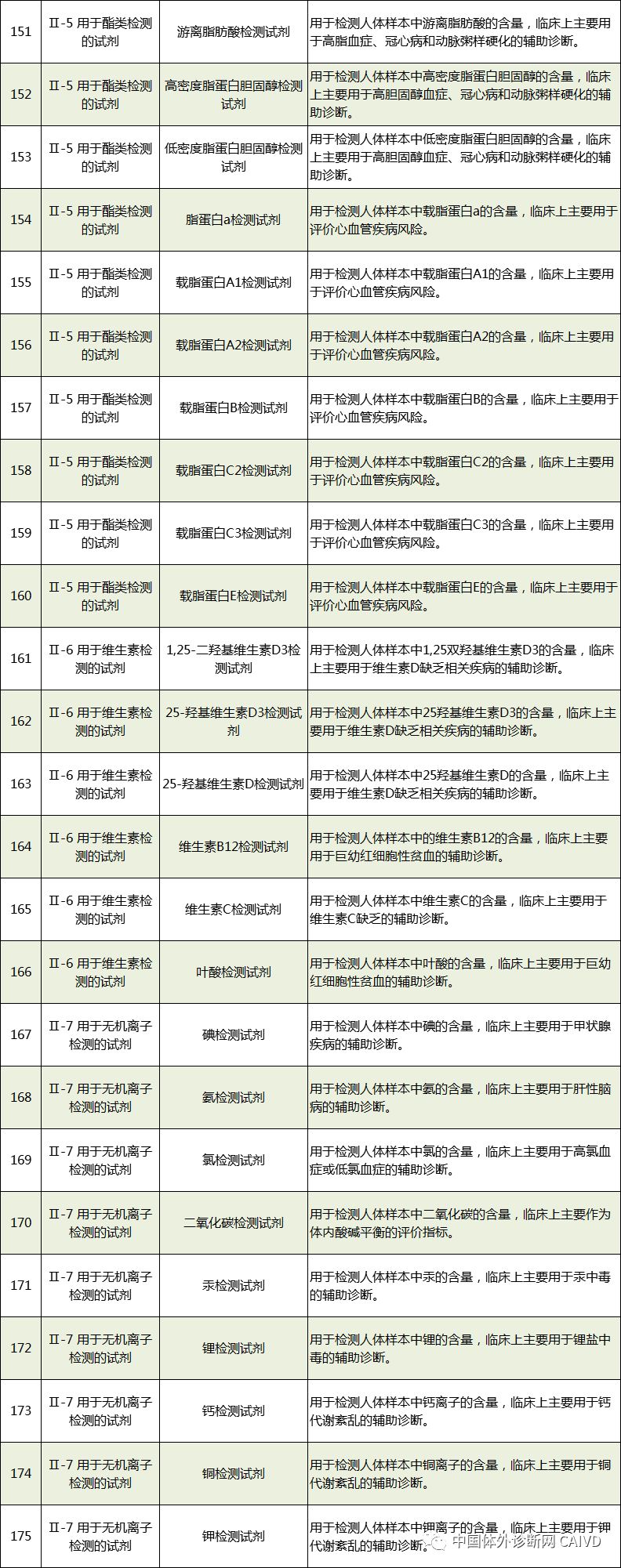
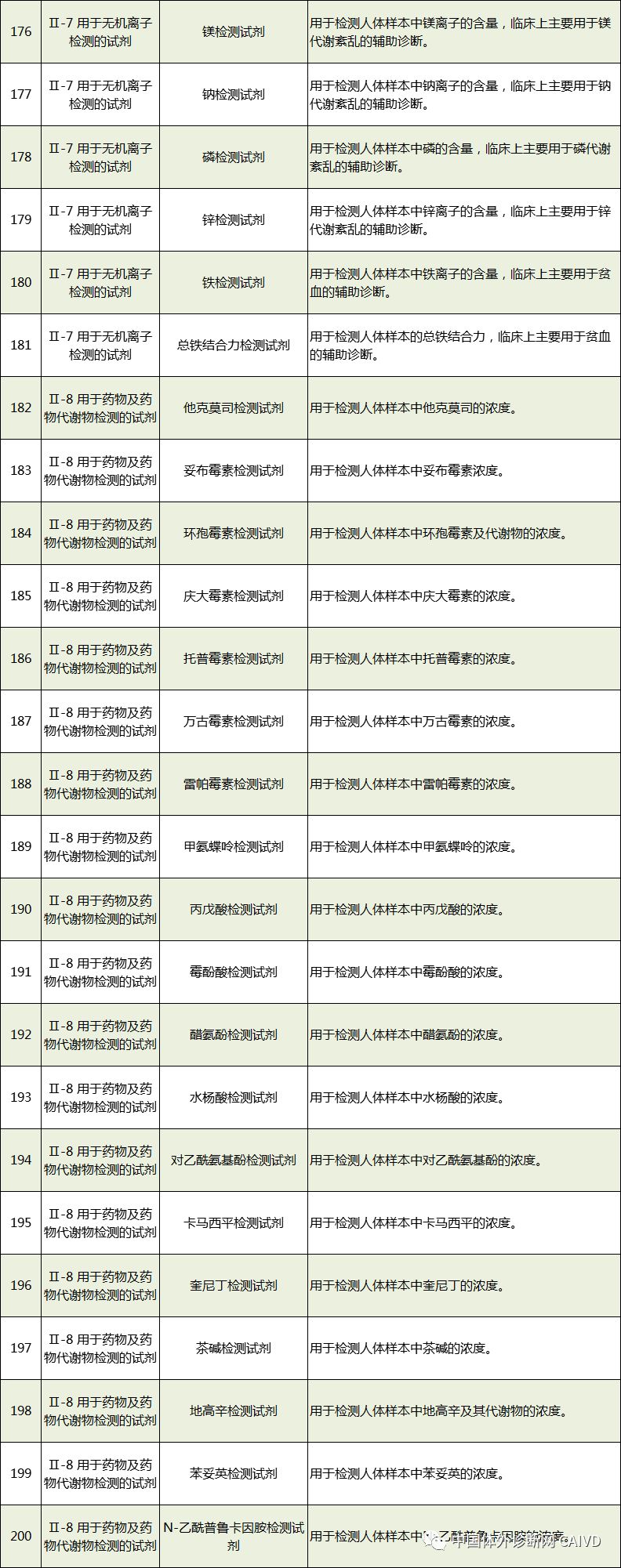
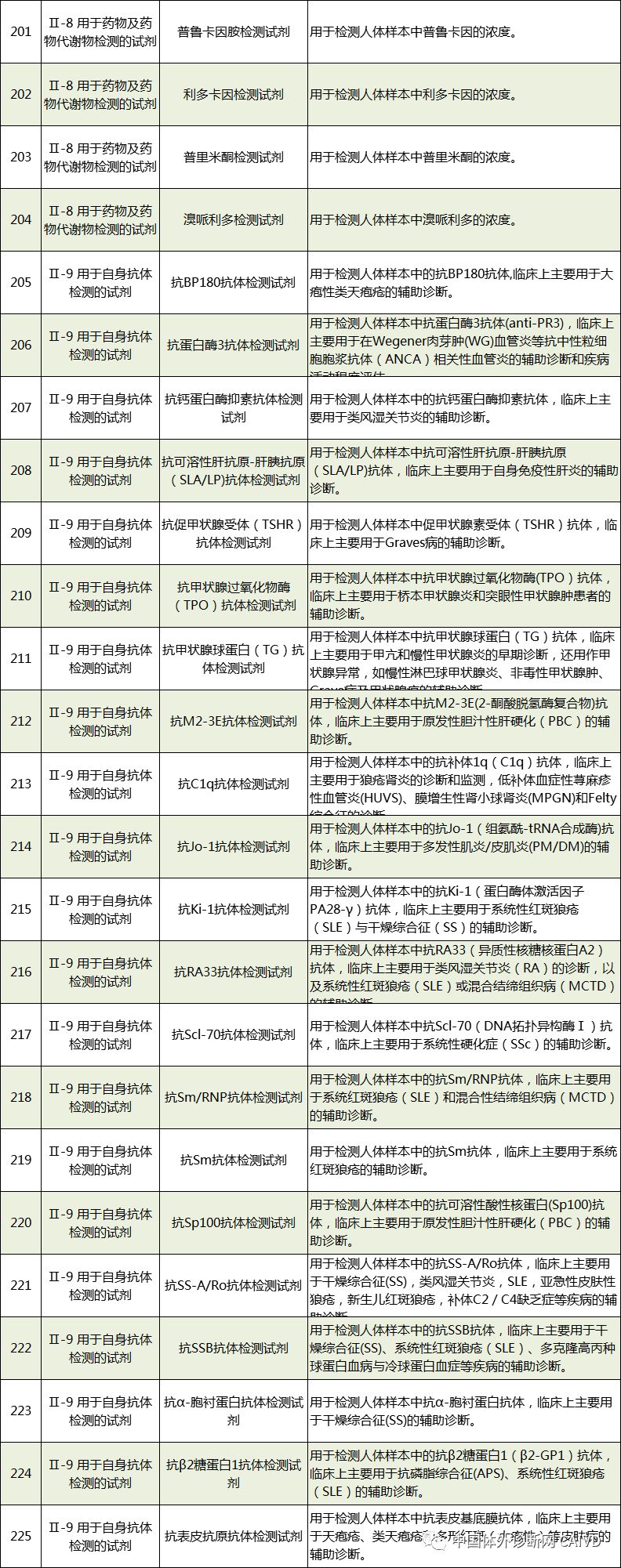
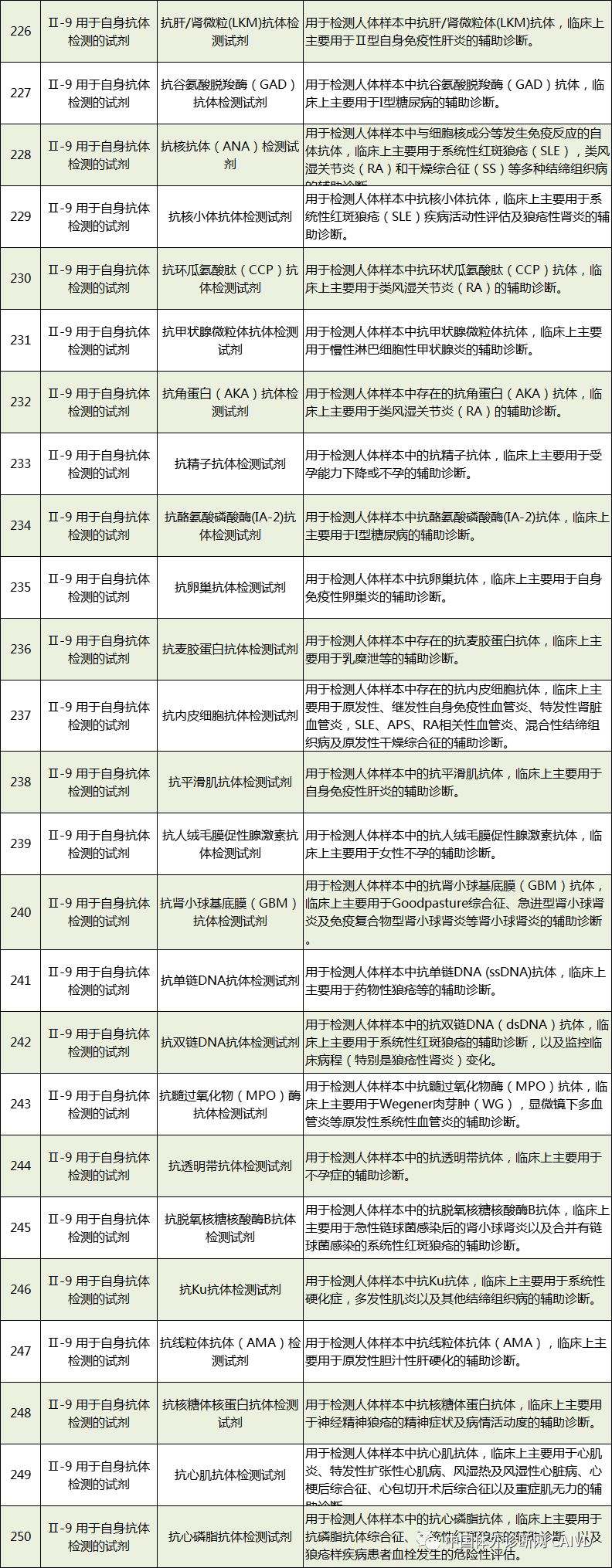
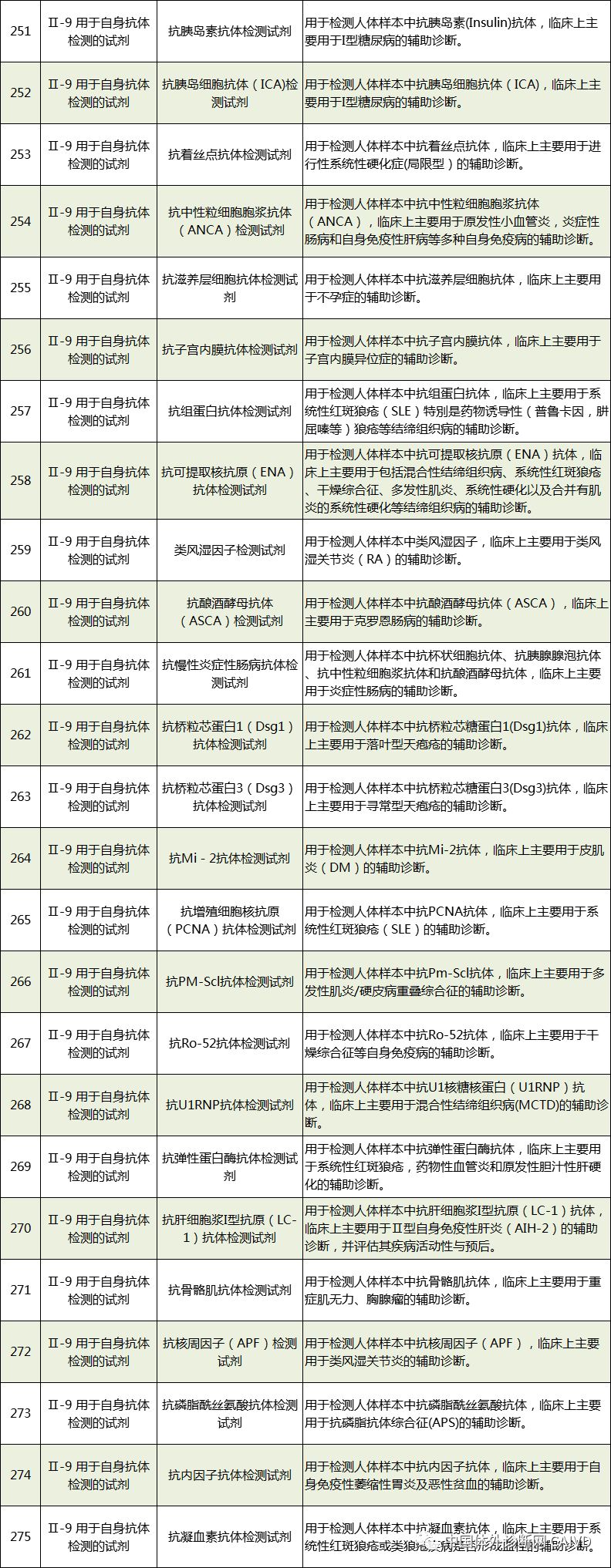
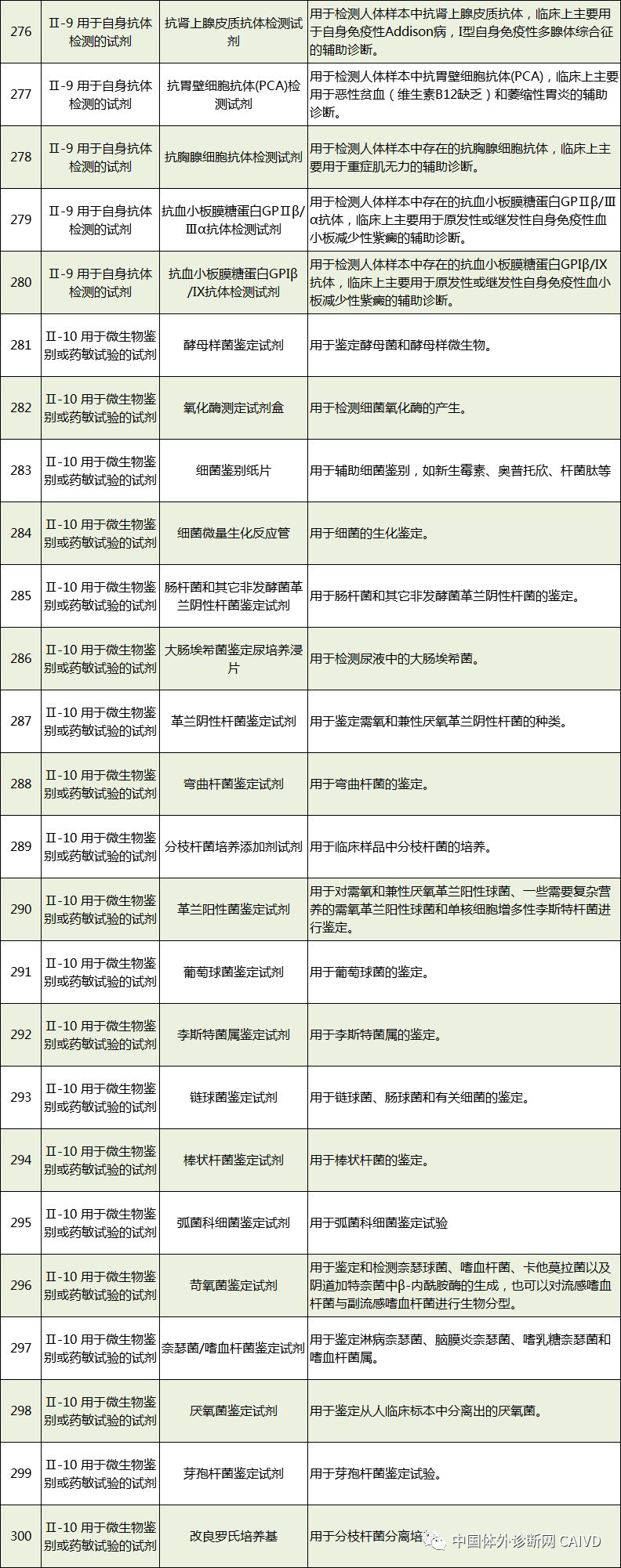
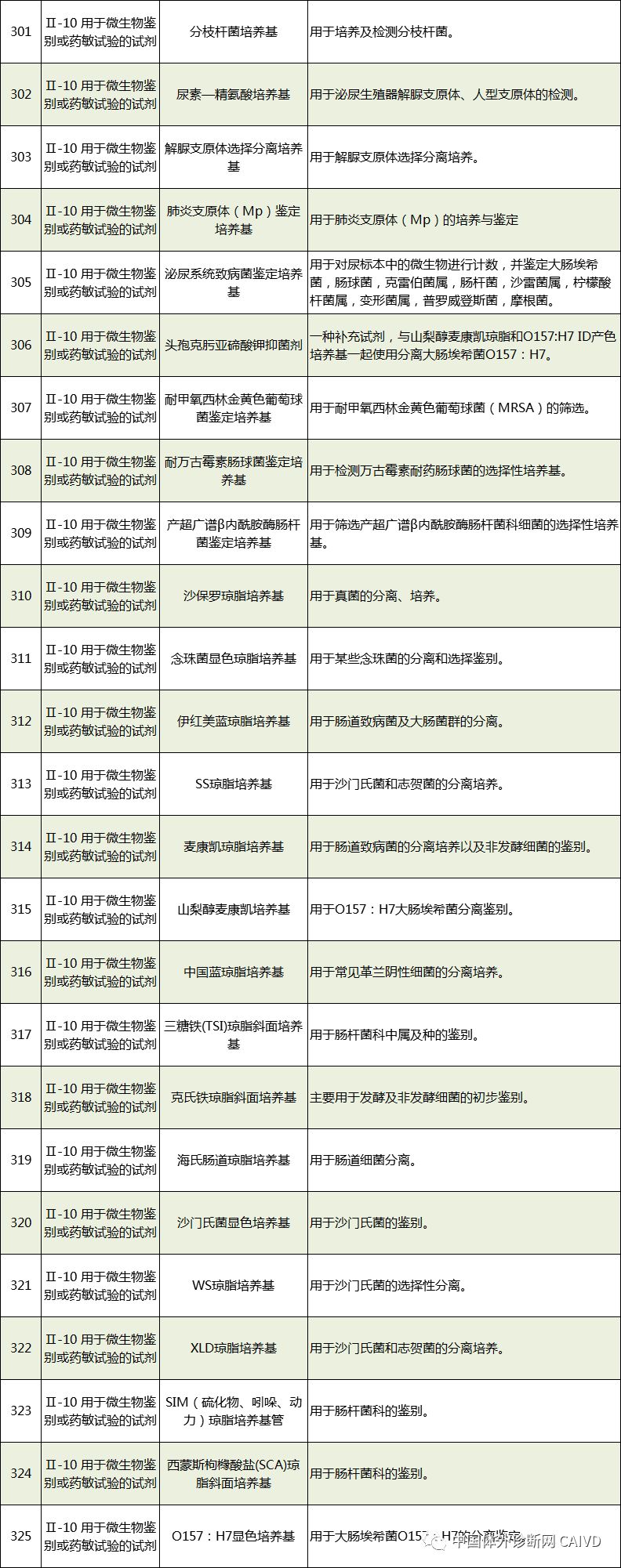
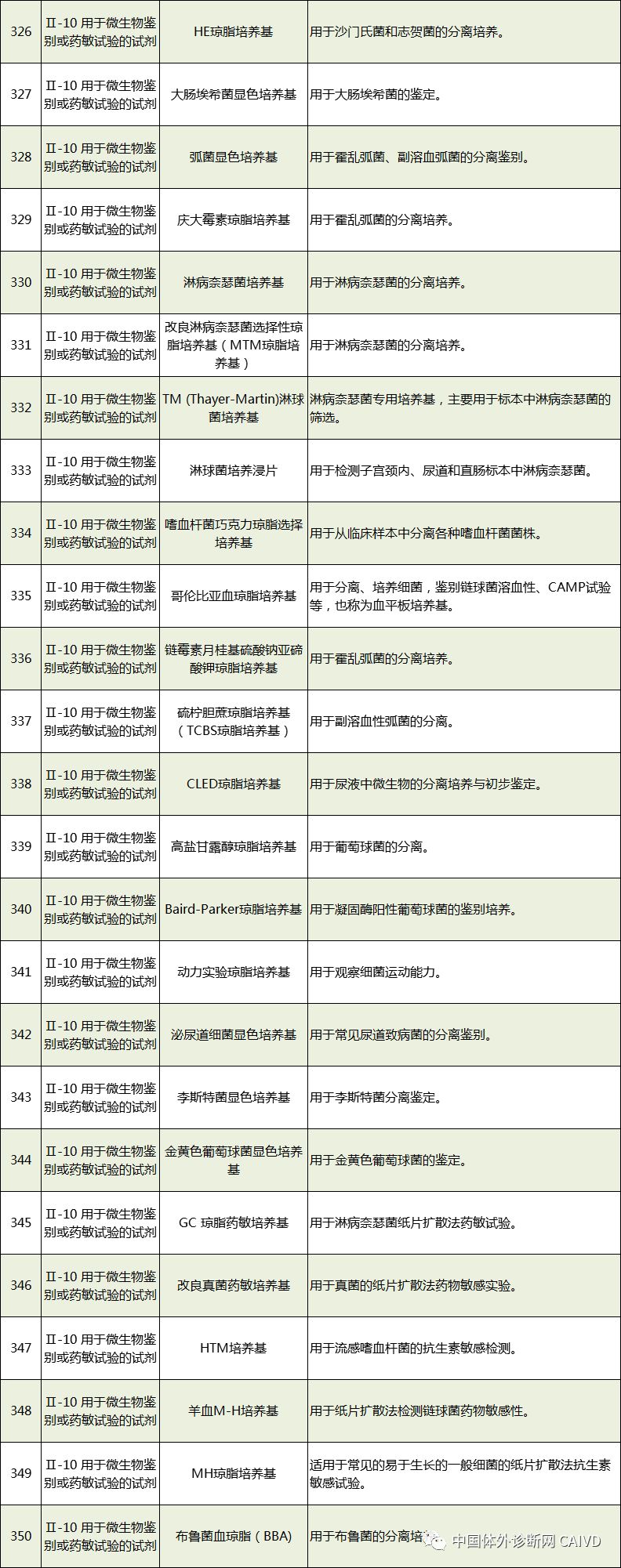
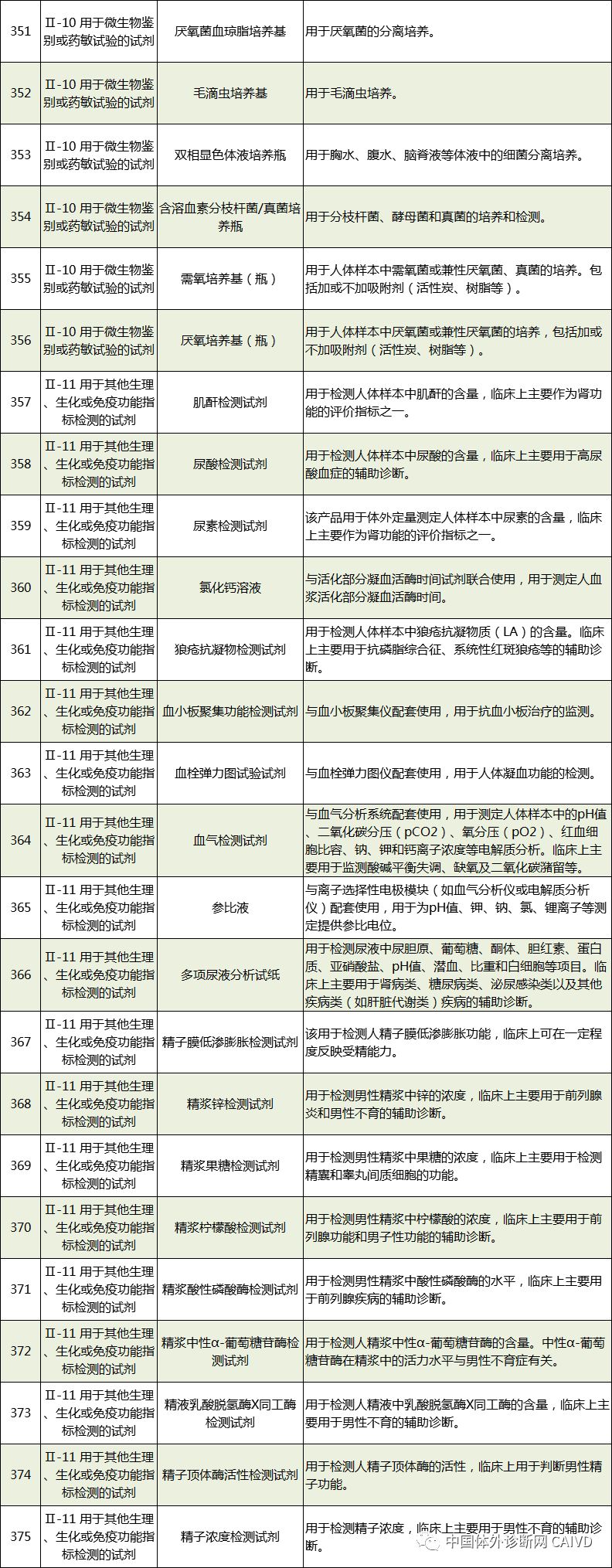
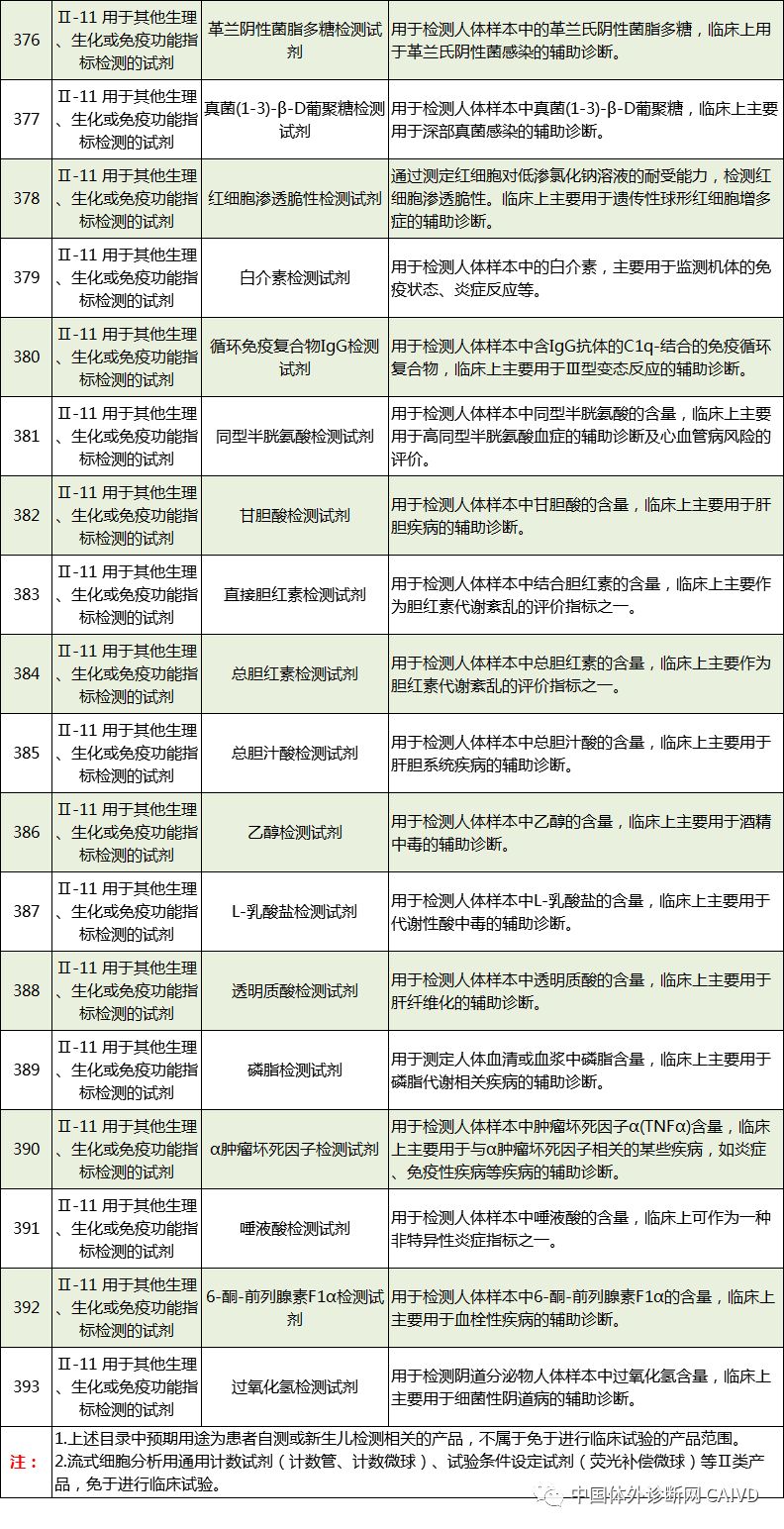
Source: National Medical Products Administration
Contributed and edited by: Yishui
Proofread by: Bonnie
Editor: ELVA

Recommended Reading
▶ Interview with a Leader: Dr. Jiang Zhiming, Global Vice President and General Manager of Beckman Coulter China
▶Interview with a Leader: Dr. Chen Lili, Chairman and General Manager of Wuhan Mingde Biotechnology Co., Ltd.
▶ Song Haibo: Current Status and Thoughts on Key Raw Materials for In Vitro Diagnostics in China
▶ Summary of Import Registration Information for Class I Medical Devices in the First Half of 2019
▶ Summary of In Vitro Diagnostic Related Diagnosis and Treatment Norms in 2018
▶Another In Vitro Diagnostic Related Association Established (with a list of 44 National In Vitro Diagnostic Related Associations)
